Few therapies for adenomyosis are available for infertile patients with reduced ovarian reserve. Previous studies investigating dopamine agonists on endometriosis have reported a reduction of peritoneal endometriosis and nerve fiber density. The present authors hypothesized that this treatment could be applied to patients with adenomyosis. They describe three infertile patients who were treated with dopamine agonists for adenomyosis. All three patients had severe dysmenorrhea due to adenomyosis with a numerical rating scale (NRS) of 10. Patients were treated twice daily with 2.5 mg bromocriptine or twice weekly with 0.5 mg cabergoline for a duration ranging from four weeks to four months. After treatment, the NRS of dysmenorrhea was about 1~2 and all patients became pregnant via in vitro fertilization-embryo transfer. Dopamine agonist acts as neurotransmitter that binds to the D2 receptor on endothelial cells and promotes VEGFR-2 endocytosis, thereby reducing immature blood vessels, nerve fiber density, and resident macrophage of endometriosis.
Adenomyosis is a benign gynecologic disease in which the endometrial stroma and glands exist within the myometrium. Symptoms typically associated with adenomyosis include excessive or prolonged menstrual bleeding, dyspareunia, and dysmenorrhea [1]. In the infertile population, the prevalence of adenomyosis is variable and has been reported to range from 8% to 27% [2]. Several reports have demonstrated a relationship of adenomyosis with pelvic endometriosis and infertility [3]. In a recent systematic review, the likelihood of clinical pregnancy following in vitro fertilization/intra-cytoplasmic sperm injection was found to be 28% lower in women with adenomyosis than in those without adenomyosis [4].
Although hysterectomy is the definitive treatment for adenomyosis, a less invasive management approach needs to be considered as first line treatment for young women who desire future fertility. Non-steroidal anti-inflammatory drugs, oral contraceptives, progestin, danazol, and gonadotropin releasing hormone (GnRH) agonists can be effectively used to alleviate symptoms, and uterine artery embolization and adenomyomectomy are effective. However, most of these treatments may cause some period of subfertility [5].
Bromocriptine and cabergoline are ergoline/ergot derivatives that act as potent dopamine D2 receptor agonist and are usually prescribed to treat prolactinemia, premenstrual syndrome, acromegaly, and Parkinson disease, as well as to prevent ovarian hyperstimulation syndrome (OHSS). There is no evidence that this drug interferes with the normal reproductive hypothalamo-pituitary-ovarian (HPO) hormonal system. Other facets of dopamine agonist activity are being studied, particularly in treating endometriosis. One previous study described reduction in the size of human peritoneal endometriotic lesion, by repeated laparoscopy of the same patients after dopamine agonist administration [6]. In another study, the effect of dopamine agonist on the reduction of endometriosis-related nerve fiber was demonstrated using a murine model [7].
With this encouraging precedent of previous studies, the idea of treating adenomyosis with dopamine agonist in infertile women emerged, since the disease characteristics and treatment methods of endometriosis and adenomyosis are similar. Here the authors describe a case series of three patients with infertile adenomyosis who exhibited remarkable improvement in dysmenorrhea by dopamine agonist treatment, and eventually became pregnant via in vitro fertilization-embryo transfer (IVF-ET).
Patient 1 first visited the clinic in 2006 for infertility. At that time, the patient was 32 years of age, had been married for six years with no children, and had one spontaneous abortion. The patient had undergone a laparotomy for an adnexa cyst at the age of 24 years, and had no other systemic diseases. Before her visit, she had unsuccessfully undergone intrauterine insemination four times and IVF once. Although her menstrual cycle was regular, she complained of severe menstrual cramps and heavy menstrual bleeding. The ultrasonogram showed a uterus measuring 9.8×4.2 cm. Then the patient was lost to follow up.
She revisited the fertility center in May 2011, at the age of 37 years. Results of a hormone assay performed as part of an infertility work up were as follows: anti-Müllerian hormone (AMH) level, 0.20 ng/mL, follicle-stimulating hormone (FSH) level, 21.85 mIU/mL, and luteinizing hormone (LH) level, 8.64 mIU/mL. The prolactin level was 4.73 ng/mL, and the estradiol level was 36.8 pg/mL. Results of the semen analysis were normal. Based on ultrasonogram, the antral follicle count was less than 10 and diffuse adenomyosis was observed (Figure 1). She had a very poor ovarian reserve. For this reason she chose not to delay the IVF trial.
 Figure 1.
Figure 1.— Case 1: sonograms of the enlarged uterus and gestational sac at a gestational age of eight weeks.
Before dopamine agonist treatment, from May 2011 to December 2012, the patient attempted 13 cycles of IVF, including seven stimulated cycles and six natural cycles, and three ETs. Pregnancy was not achieved. Dopamine agonist treatment was administered from January to March 2013. A dose of 1.25 mg of bromocriptine was prescribed twice daily for 30 days. Then, 2.5 mg of bromocriptine was prescribed twice daily for another 30 days. After the dopamine agonist treatment, she continued her fertility treatment and underwent one stimulated cycle, six natural cycles, and three ETs until she finally achieved pregnancy (Figure 1) by a frozen-thawed ET of three embryos conducted on December 2013. Her endometrium was 0.7 cm thick and the grades of the embryos were 10CG1, 10CG2, and a morula. This pregnancy ended with a spontaneous abortion at nine weeks of gestation. However, her dysmenorrhea was greatly improved after the dopamine agonist treatment, from an NRS of 10 to an NRS of 1~2, as well as decreased menstrual flow. However the size of her uterus was not reduced.
This 37-year-old patient first visited this center in August 2015 and she had been married for four years. She had previously undergone two cycles of IVF at another fertility center and they were all unsuccessful. Before these IVF cycles, she had also had two spontaneous abortions. She had taken medication for tuberculosis for six months when she was 31-years-old. Her period interval was irregular and ranged from 60 to 160 days. The amount of menstruation was profound and the pain was severe. An ultrasonogram indicated that her uterus was enlarged to 8.44×6 cm (Figure 2). Her hormonal profile was as follows: AMH level, 7.69 ng/mL, FSH level, 8.23 mIU/mL, and prolactin level, 10.67 ng/mL. Results of the semen analysis were normal.
 Figure 2.
Figure 2.— Case 2: sonograms of the enlarged uterus and gestational sac at a gestational age of 10+2 weeks.
She agreed to undergo treatment with a dopamine agonist before other treatments for adenomyosis. She began her medication from the first day of her visit. She was prescribed 1.25 mg of bromocriptine once daily for a week and twice daily for another week. Then, her first IVF cycle began. Twenty-four eggs were retrieved and 20 ova were fertilized; all 12 viable embryos were frozen to prevent OHSS. After ovum retrieval, she began taking 0.5 mg of cabergolin twice per week for two weeks. During her next cycle, three embryos were transferred, and she achieved a pregnancy (Figure 2). The endometrium was 0.9 cm before ET, and the grades of the embryos were 12CG1, 12CG2, and 7CG3. She gave live birth by cesarean section at 38 weeks. This patient also reported a dramatic improvement in dysmenorrhea following treatment with the dopamine agonist. Her NRS decreased from 10 to 1 when she started her period after the second week of treatment. The size of her uterus was not reduced after dopamine agonist treatment.
This patient first visited this center in February 2016. At that time, she had been married for 3 years and was 33 years of age. Before the visit, she had undergone treatment with three cycles of intrauterine insemination and one cycle of IVF at another center. She had never been pregnant and had no specific medical history. Her interval of menstruation was irregular from 30 days to 90 days with menorrhagia and dysmenorrhea. On the ultrasonogram taken on the first visit, her uterus was enlarged to 7.7 cm × 17.5 cm with adenomyosis and multiple myomas (Figure 3). Her hormonal profile was as follows: AMH level, 5.59 ng/mL, FSH level, 4.64 mIU/mL, prolactin level, 7.50 ng/mL, and estradiol level 45.6 pg/mL. Semen analysis indicated that semen motility was decreased to 33%.
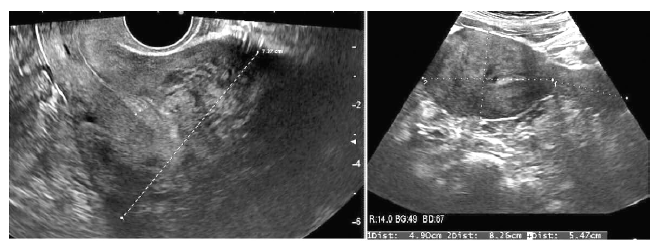 Figure 3.
Figure 3.— Case 3: sonograms of the uterus before and after D2 agonist and gonadotropin releasing hormone agonist treatment. The size of the uterus is significantly reduced after the treatment.
She was prescribed bromocriptine from the first day of her visit for 4.5 months; 1.25 mg once daily for a week, twice daily for another week, and then the dosage was adjusted to 2.5 mg twice daily for 120 days. She was simultaneously treated with three cycles of the GnRH analog. After treatment, her dysmenorrhea was relived from an NRS of 10 to an NRS of 1, and the size of her uterus was reduced to 4.9×13.7 cm (Figure 3). Laparoscopic myomectomy and tuboplasty, and hysteroscopic polypectomy were performed before initiating IVF. Five months postoperatively, IVF was conducted and 12 eggs were retrieved, of which four were fertilized. Among them, two embryos were transferred, resulting in pregnancy. The endometrium thickness was 1.5 cm before ET, and the grade of the embryos was 8CG1. Unfortunately, a spontaneous abortion occurred at the gestational age of 10+3 weeks.
There have been several studies reporting that adenomyosis may impair fertility. Additionally, adenomyosis is the most common disease related to severe menstrual cramping along with endometriosis. These two diseases originate from ectopic endometrial tissue, and the treatment for symptoms of adenomyosis follows the same protocol as for endometriosis. Endometriosis is a chronic inflammatory gynecologic disease that induces dysmenorrhea and pelvic adhesion.
The inflammation is associated with resident macrophages, mast cells, and fibroblasts, all of which produce proangiogenic factors [8, 9]. The vascular endothelial growth factor (VEGF) which is produced by macrophage, induces angiogenesis of immature blood vessels, stimulates nerve-fiber growth, and increases nerve fiber density in peritoneal endometriotic lesions [10]. The nerve growth factor, which is involved in neuronal proliferation, survival and path-finding also induce angiogenesis through crosstalk with the VEGF, and it has been reported that the neuronal and vascular cells reciprocally affect their own growth via paracrine mechanisms and common signaling [11, 12]. The nerve fiber density in the endometriotic stromal cells in peritoneal lesions is directly related to chronic pelvic pain and dysmenorrhea [13]. It has been reported that women with endometriosis exhibit significantly increased nerve fibers density in the basal layer of the endometrium and myometrium [14].
The dopamine neurotransmitter binds to dopamine receptor-2 located on the endothelial cell surface, and promotes VEGF receptor-2 endocytosis, thereby preventing VEGF-VGEFR-2 binding. The receptor phosphorylation is blocked, and the angiogenesis process is inhibited. Consequently, blood vessel regression occurs and neuroendocrine and immune disequilibrium takes place in the endometriotic tissue. Using a murine model, researchers have shown that dopamine agonist treatment reduces the amount of immature blood vessels, nerve fiber density, macrophage and the mast cell count, and thus the size of the endometriotic lesion and extensiveness of the inflammatory milieu of endometriosis [7].
In another study, patients with endometriosis and hyperprolactinemia who were treated with quinagolide, a dopamine agonist, which exhibited a decrease in the size of peritoneal endometriotic lesion. In particular, quinagolide induced a 69.5% decrease in the size of the lesions, with 35% of patients experiencing complete lesion disappearance. Furthermore, after administering quinagolide, the production of VEGF decreased, and the density of the VEGF R-2 decreased by half [6].
To the best of the current authors’ knowledge, this case series is the first to report adenomyosis under treatment with a dopamine agonist. Although the number of cases in this series was small, all three patients exhibited a reduction in pain and achieved pregnancy. This encouraging result opens a new possibility to employ dopamine agonists to treat infertile women with adenomyosis. Attention should be paid to the fact that the dopamine agonist allows the continuation of IVF in patients with severely poor ovarian reserve and intractable dysmenorrhea due to adenomyosis, as observed in the first patient. Furthermore, to enhance the efficacy of treatment, it can be used prior to or concurrently with other treatments for adenomyosis, as seen in the third patient. However, the effect of the treatment modality described herein may vary across individuals and methods. Accordingly, further studies in a larger population are needed to definitively understand the efficacy of treatment, appropriate dosage, treatment period, and criteria of patients who would most benefit from treatment.
The dopamine agonist, which has long history of treating various medical conditions has anti-angiogenic effect and three case patients have been successfully treated for severe dysmenorrhea due to adenomyosis. Further studies in a larger population are required.
