One of the rarest forms of ectopic pregnancy is represented by the implantation of pregnancy on the scar of cesarean section. The implant in the uterine scar tissue exposes the patient to a risk of massive bleeding, uterine rupture, and penetration by the syncytiotrophoblast into the bladder wall. Currently, there is no consensus on the optimal management of this condition. The medical, surgical, and radio-interventional approaches are all contemplated. Here the authors report and discuss the diagnosis and treatment of three cases of caesarean scar pregnancies.
Ectopic pregnancy is a major cause of maternal mortality during the first trimester of pregnancy, with a prevalence estimated in 8 per 1,000 pregnancies [1, 2].
Non-tubal ectopic pregnancies are uncommon, accounting for about the 5% of all the ectopic pregnancies. The possible sites of non-tubal ectopic implantation are cesarean scar, cervix, interstitium, cornual region, ovaries, and other pelvic organs [2-4].
Scar ectopic pregnancy is becoming increasing common all over the globe. It may occur following cesarean section (i.e. the major risk factor for scar pregnancy, with a risk of 0.15%), myomectomy, endometrial curettage or operative hysteroscopy [5, 6].
Symptoms of ectopic pregnancy are subtle and include diffuse or localized pelvic pain, shoulder tip pain, vaginal bleeding, diarrhea, nausea, and vomiting. Nevertheless, scar pregnancies are often diagnosed in asymptomatic women [2, 4, 7].
A timely diagnosis and therapy of ectopic scar pregnancies allows to avoid severe complications such as uterine rupture and hemorrhagic shock. Nevertheless, the management of this condition is still not standardized [3, 6, 8, 9].
Here the authors report three cases of cesarean scar pregnancies whose prompt identification and treatment allowed the prevention of severe complications.
A 34-year-old parous patient with a previous history of caesarean section due to fetal distress referred to this University Hospital with a positive pregnancy test (B-hCG: 5,100 mIU/mL), at six weeks and four days of amenorrhea. The patient, on inspection and specular examination, presented neither blood loss nor pelvic pain. At transvaginal ultrasound examination, an anechoic formation of 18 mm was identified at the isthmic level, in correspondence of the previous cesarean scar. The patient was admitted to this University Hospital for a suspect of ectopic pregnancy. A repeat ultrasound examination, after 48 hours, confirmed the presence of a gestational camera equipped with a yolk sac. The B-hCG value was 9,300 mIU/mL. The patient was treated with methotrexate 1 mg/kg i. m. and subsequently the pregnancy was removed by resectoscopic hysteroscopy. Approximately 24 hours after surgery, the B-hCG values showed a marked decline. Then, the patient was discharged (on the same day) and followed-up through monitoring B-hCG values, until their negativization. An ultrasound examination confirmed the resolution of the condition two weeks later. The histological examination confirmed the presence of deciduous material. The patient did not experience any type of medical or surgical complication.
A 37-year-old, parous patient with a previous delivery by cesarean section was admitted to this University Hospital due to diagnosis of retained abortion at eight gestational weeks. The patient was asymptomatic and the obstetric visit was negative, without signs of vaginal blood losses. Transvaginal ultrasound showed a gestational sac with an average diameter of 29 mm and an embryo with CRL of 15 mm without cardiac activity. The implant was located at the uterine isthmus, on the previous cesarean section scar. Vaginal administration of an ovum of cervidil was undertaken. After a single administration, the patient began to feel moderate pelvic pain associated with vaginal bleeding. At that point, the authors proceeded with a surgical evacuation of the uterine cavity. The patient underwent an ultrasound-guided (with transabdominal probe) endohysterosuction. The procedure was completed with a delicate curettage by using a curved curette. The patient did not experience complications after surgery. The post-intervention B-hCG assay showed rapidly falling values in the days following the previous findings.
A patient of 22 years, parous, who previously (seven months before referring at this University Hospital) underwent cesarean section at the 37th week of pregnancy due to fetal tachycardia. She referred to thus emergency-obstetric room due to severe pelvic pain and amenorrhea at eight weeks. Serum B-hCG values were 51,388 mUI/mL. At transvaginal ultrasound, an anechogenic, nonhomogeneous lesion, compatible with a dysmorphic gestational chamber, in the isthmic region was found. This formation was protruding across the anterior uterine wall until the posterior bladder (Figures 1 and 2).
 Figure 1.
Figure 1.— Transvaginal ultrasound sagittal scan: the scar pregnancy, which protruding out of in bladder, shows a rich vascularization at color Doppler examination.
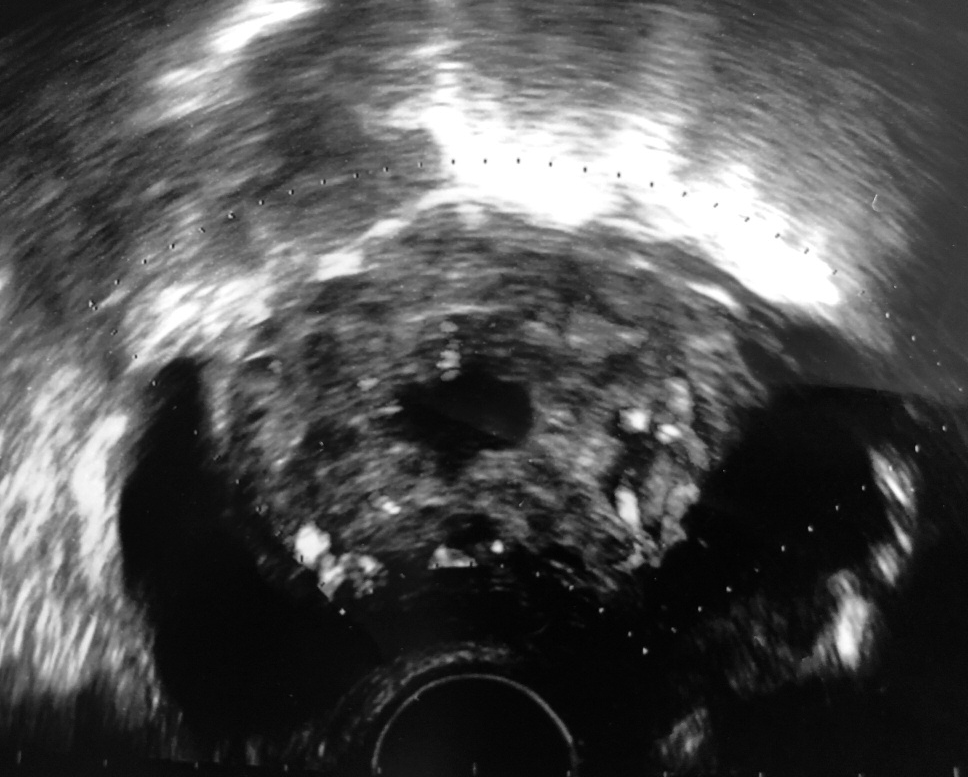 Figure 2.
Figure 2.— Transvaginal ultrasound transversal scan: the scar pregnancy, which protruding out of in bladder, shows a rich vascularization at color Doppler examination.
On the admission day, a diagnostic cystoscopy was performed, showing a formation which imprinted the bladder cavity between the two ureteral outlets. At hysteroscopic examination, the authors found the presence of trophoblastic-like material at the level of the anterior isthmic region. A pelvic NMR showed the presence of a rough roundish formation with a diameter of about 55 mm that was protruding in the left lateral pelvic area from the lateralposterior-inferior side of the uterus body. The anterior uterine wall, at this level, showed a wide parietal interruption, in communication with the endometrial cavity. The lesion appeared as vacuolar, with numerous convoluted and lacunar vascular formations. The picture was compatible with an ectopic gestural sac located on the scar of the previous caesarean section. Subsequently, further 2D and 3D ultrasound scans were performed, confirming the diagnosis. In consideration of the peculiar case (young age and desire for future pregnancies), the authors decided to treat the patient by administering the following therapeutic regimen: methotrexate 1 mg/kg intramuscular injection on days 1, 3, and 5 and levofolinate calcium 0.1 mg/kg intravenously on days 2, 4, and 6. After therapy, the B-hCG values were equal to 34,186 mIU/mL. The control ultrasound survey showed a slight reduction in the size of the previously described formation. A reduction of the vascular gaps surrounding the gestational sac was found at Doppler colour ultrasonography. Therefore, the authors decided to proceed with a repeat cycle of MTX plus levofolinate calcium, during which a severe anemization of the patient occurred, necessitating for blood transfusion. After the second cycle of methotrexate, the B-hCG values were 18,300 mIU/mL and the ultrasound examination showed a volumetric reduction of the formation, which appeared of 43 mm in its maximum diameter. At this point, the authors opted to proceed with surgical treatment. On the same day, the patient underwent laparotomic surgery. The portion infiltrated by the trophoblast was removed by curettage. The morphology of the uterine wall was successfully reconstructed, in order to preserve future fertility. The procedure was completed without major complications. A control MRI performed nine days after the surgery showed a complete restoration of the uterine anatomy. A rapid decrease in serum B-hCG was observed, reaching 10.4 mIU/ml at 30 days from surgical treatment. The patient was discharged from hospital on the 11th day after surgery, and an oral contraceptive therapy was administered for three months.
The ectopic implant of an embryo on cesarean scar is an event whose prevalence is difficult to assess, as only a few number of case reports are published [9, 10]. Notably, in the last decade, there has been a substantial increase in the number of reports in this regard, perhaps due an increase of pregnancies in women with a history of caesarean section [5, 11]. Additionally, in the last two decades, there has been a considerable increase in pregnancies on hysterotomic scar following embryo-transfer, this derived from the proportional increase in the use of medically assisted fertilization techniques [7-9, 12, 13].
Cesarean scar pregnancy was first described in 1978 by Larsen and Solomon [9], who emphasized its variable clinical presentation. The patient with a scar pregnancy may have a severe clinical condition (with significant blood loss associated with intense pelvic pains), or may be completely asymptomatic. Nevertheless, the authors must stress that it rarely progresses as asymptomatic up to ten gestational weeks or more [10, 14]. The diagnosis of cesarean scar pregnancy is usually made by ultrasound, generally with a transvaginal probe and color flow Doppler. Rarely, more invasive methods (such as office hysteroscopy) may be required [15, 16].
A significant factor to keep in mind is the dating of the pregnancy, referable to the average diameter of the gestational camera and to the CRL value. The greater the week of amenorrhea, the value of the average diameter of the gestational chamber and of the greater CRL, the major will be infiltration of the trophoblast into the thickness of the myometrium and, depending on the case, even externally to it, involving structures outside the uterine serosa [17].
The portion of the uterine wall, involved in the hysterotomic access of the previous cesarean section, has a reduced thickness compared to a uterus on which surgery has never been applied [3, 7]. This condition is the determining factor that produces the high risk of complications for this given implant location. The pregnancy, in this condition, goes to implant itself on a thinned and fibrotic tissue that borders directly with the inferior bladder wall [8, 9]. Given the infiltrating capacity of the trophoblast and the functional anatomical features, both dynamic and static of the hysterotomic scar, they cause the damage produced to be decidedly dramatic [5, 6].
The risk to which patients with this type of implant are exposed varies, including bleeding, uterine rupture, bladder infiltration, up to the necessary hysterectomy, with consequent injured function regarding future fertility [18]. Regarding the treatment of the case, given the rarity of the disease, to date there are no guidelines for its optimal management. We can choose between various therapies: medical, surgical, and interventional radiology [11, 13, 17-20].
Medical treatment involves the use of systemic methotrexate and/or ultrasound-guided injection within the gestational chamber of different agents (methotrexate, KCL, vasopressin, hyperosmolar glucose, etc.) to interrupt the pregnancy [21-23]. Methotrexate is the cornerstone of conservative therapy.
The surgical approach is designed to mechanically remove the pregnancy. In this regard, the rational involves the excision of the trophoblast from the myometrium via laparotomy and endoscopy (hysteroscopic resectoscope, laparoscopic) [24-26]. In cases where pregnancy has produced extensive infiltration, hysterectomy and repair of any bladder damage is also considered if trophoblastic infiltration has spread outside the uterus [27, 28]. On other hand, interventional radiology has the considerable advantage of reduced invasiveness [29]. The mentioned interventions, especially the surgical ones, can create a reduction in fertility, exposing patients to a greater risk of uterine rupture in subsequent pregnancies and relapses [12, 28, 29]. These risks are currently not quantifiable due to the lack of data present in the literature.
It is important to underline how often, in clinical practice, single therapeutic methods described are not always decisive. Sometimes it is necessary to refer to more than one method to obtain a complete resolution of the clinical picture.
The patients whose clinical course is reported did not present any short-term complications. The patient referable to the second case is currently pregnant.
In conclusion, cesarean scar pregnancy represents a rare and life-threatening complication of the first trimester of pregnancy. Clinical history, ultrasound examination and serum B-hCG measurements are mandatory for a prompt diagnosis. Additional diagnostic techniques, such as cystoscopy, hysteroscopy, and MRI, may be helpful to better assess the trophoblast’s infiltration (across the uterine wall) and invasion of surrounding structures (such as bladder).
A timely intervention, including medical and/or surgical therapy, is often conclusive. The therapeutic approach varies according to the clinical scenario, the experience (and skill) of the operator, and the patient’s desire for future pregnancies. As current management of cesarean scar pregnancy is not standardized, future multicentric studies are needed to provide evidence on the best therapeutic approach for this condition.
Ph. Doctor School in Biomedical Sciences, Address in Gender Medicine, Men, Woman and Child, Sassari University, Italy, supported the study.
