Purpose: Evaluation of the effect of platelet-rich plasma
(PRP) containing platelet-derived growth factor on in vitro fertilization (IVF)
failure patients. Methods: In this clinical trial, 80 eligible
patients (infertile women with at least two IVF failures) were randomly assigned
into two groups, including patients who received an intrauterine infusion of PRP
(n: 40) and controls (n: 40). Before PRP therapy, standard hormone replacement
therapy was performed for all patients to endometrial thickness preparation.
After sonographic assessment of endometrium, PRP was injected into all patients’ endometrium whether they had an appropriate endometrial thickness or not. Then, the embryo transferring was done through IVF. Eventually, the consequences of
fertility, embryo implantation, and pregnancy were evaluated. Data analysis was
performed using SPSS version 22. Results: The rate of successful IVF
[6 (15%)], pregnancy rate [5 (12.5%)], and live birth [5 (12.5%)] were relatively
higher in patients undergoing PRP therapy compared with controls [2 (5%), 1
(2.5%) and 0, respectively]. However, their statistical difference was not
significant between the two groups (P
The successful fetus implantation requires the proper growth of the fetus simultaneously with an appropriate receptive endometrium. The thin endometrium is not responsive to standard treatments, and it’s still a challenge for assisted reproductive techniques (ART) which usually leads to cycle cessation and unplanned embryo freezing [1, 2]. So far, various strategies have been developed for the treatment of thin endometrium, including the long-term exogenous estrogen therapy [3], use of low dose aspirin [4], vitamin E [5], sildenafil citrate (vaginal) [6], electroacupuncture [7], and use of granulocyte colony-stimulating factor (G-CSF) [8]. However, the mentioned treatments are not successful in a number of women with a thin endometrium.
Several studies in different countries have investigated the therapeutic effect
of platelet-rich plasma (PRP) containing platelet-derived growth factors (PDGF)
on endometrial growth and provided significant results [9, 10, 11, 12]. PRP is prepared
from whole fresh blood containing several growth factors and cytokines that
include PDGF, transforming growth factor-beta (TGF
Previously, PRP has been studied as a treatment for several medical disorders, including nerve damages, eye epithelium defects, hair loss, myocardial damage, osteoarthritis, and tendinitis. Despite the widespread use of PRP in various medical fields, its effectiveness on obstetrics and gynecology diseases has been studied in rare studies [11, 12, 18]. Accordingly, the present study was designed to investigate the effect of PRP containing PDGF on the unsuccessful embryo implantation in women undergoing IVF, whether they had an appropriate endometrial thickness or not. The effects of PRP therapy on IVF failure patients were evaluated based on the rates of embryo implantation, successful pregnancy, and live birth. Our findings provide valuable information to improve pregnancy outcomes through the IVF process.
| Statement of Significance |
| Problem: Most cases of female infertility are faced with in vitro fertilization-failure due to the thin endometrium or other unknown reasons. |
| What is Already Known: So far, the considerable therapeutic effect of PRP containing PDGF has been shown on endometrial growth. |
| What this Paper Adds: This study evaluated the therapeutic effect of PRP in the improvement of the IVF process, pregnancy, and live birth rates. |
Eligible patients were randomly assigned into two groups, including patients who have received an intrauterine infusion of PRP and patients without PRP injection (controls). The inclusion criteria were infertile women with at least two IVF failures and age below 41 years old. The exclusion criteria were women with chromosomal, genetic, and uterine abnormalities, hematological or immunological disorders, and hormonal disorders. Also, the embryos that arise from such maternal and paternal abnormalities were excluded (Fig. 1).
 Fig. 1.
Fig. 1.Flowchart: The inclusion and exclusion criteria to select eligible infertile women.
Before PRP therapy and embryo transfer process, standard hormone replacement therapy (HRT) with Estradiol valerate (Transdermal dosage: 0.2_0.4 mg/day; Aburaihan, Iran) was performed for all patients to endometrial thickness preparation. This dose causes blood levels of 200 to 400 pg/mL which is equivalent to the normal level in the late follicular phase [19]. Eventually, the endometrial thickness reached the appropriate size (7-10 mm) in 38 patients while 2 women did not reach the appropriate size (7 mm). Endometrial thickness is measured by ultrasound from the echogenic border to the echogenic border across the endometrial cavity on a sagittal midline image.
For luteal phase support in IVF women, we used Crinone 8% Progesterone Vaginal Gel containing 90 mg of progesterone (Merck KGaA, Darmstadt, Germany) on a daily dosage of 1.125 g 8% gel, starting on the 18th - 21st day of the cycle.
After sonographic assessment of endometrial thickness, PRP (1.5 mL) was injected
into all patients’ endometrium whether they had an appropriate endometrial
thickness (7-10 mm) or not (
PRP is prepared from autologous blood by “Fertilize Lympho-PRP kit”. Firstly, 8.5 mL of venous blood was drawn from the syringe pre-filled with 1.5 mL of anticoagulant solution (ACD; Terumo; at a ratio of 10 : 1.5) and centrifuged immediately at 12,000X g for 10 min.
The blood was divided into three layers: red blood cells at the bottom, cellular plasma in the supernatant, and a Buffy coat layer between them. The plasma layer was collected to another tube and re-centrifuged at 12,000X g for 10 min. White sediment of platelet pellets was formed at the bottom of the tube that was PRP. According to the PRP kit’s instructions (PRP centrifuge kit; Prodizen, Korea), the white sediment dissolved in 1.5 mL plasma [the 1.5-line indicated on the tube for infertility cases] was used as PRP containing platelet growth factor. The IVF process is organized into four main steps as follows:
1. Stimulation/superovulation
2. Egg retrieval
3. Insemination and Fertilization
4. Embryo culture
The successful IVF and IVF failure were respectively defined as successful embryo implantation and the failure of implantation.
The “pregnancy live birth” was defined as the delivery of a live born child after 24 weeks of gestational age.
Considering the main purpose of the research, the researcher’s idea, previous
studies [20], and
N: 40
Totally: 80 N
This parallel-group, single-blind, and randomized controlled trial was randomized by designing a table of the randomized block size of 6. To maintain single-blinding, the responsible investigator was unaware of the grouping of each patient. But, the main physician and patients were aware. However, the physician did not consciously interfere in the selection of patients for each group. In fact, she started the treatment process on the advice of a second researcher, who had designed a table of the randomized block without knowing the patients’ medical conditions.
A chi-square test was used to detect a relationship between quality variables.
Also, the Mann-Whitney U test or t-test was used for quantitative variables.
Generalized Estimating Equations (GEE) were used to determine the relationship
between variables measured over a number of times during the study [Considering
P-value
This clinical trial was approved by the ethics committee of Ahvaz Jundishapur University of Medical Sciences with Ethical Code: IR.AJUMS.REC.1396.634.
All subjects gave their informed consent before participating in the study. All procedures performed in this study involving human participants were in accordance with the ethical standards of the national research committee and with the 2008 Helsinki declaration and its later amendments or comparable ethical standards. This clinical trial has been registered in the Iranian Registry of Clinical Trials (IRCT registration number: IRCT20180102038182N1).
All the demographic and clinical data of patients are presented in Table 1.
Also, Table 1 presents the results of the statistical comparison of all variables
in both the PRP
| Variables | All women Mean |
PRP |
PRP |
P-value |
| Age (years) | 31.9 |
34.15 |
32.82 |
0.33 |
| Marriage duration | 8.52 |
8.82 |
8.22 |
0.9 |
| Infertility duration | 7.22 |
7.5 |
6.95 |
0.85 |
| Time elapsed since the first IVF* | 1.66 |
1.96 |
1.36 |
0.06 |
| Educational status: | ||||
| Degree level under high school diploma | 49 (61.3%) | 24 (60%) | 25 (62.5%) | |
| High school diploma or upper levels | 31 (38.7%) | 16 (40%) | 15 (37.5%) | |
| BMI | ||||
| 59 (73.75%) | 28 (70%) | 31 (77.50%) | 0.612 | |
| 21 (26.25 %) | 12 (30%) | 9 (22.50%) | ||
| Type of infertility | ||||
| Primary | 66 (82.55%) | 34 (85%) | 32 (80%) | 0.7695 |
| Secondary | 14 (17.5%) | 6 (15%) | 8 (20%) | |
| Cause of infertility: | ||||
| Male related causes | 25 (31.3%) | 12 (30%) | 13 (32.5%) | 0.901 |
| Female related causes | 23 (28.8%) | 11 (27.5%) | 12 (30%) | |
| Both causes | 32 (40%) | 17 (42.5%) | 15 (37.5%) | |
| The number of IVF full cycles | ||||
| 2 | 58 (72.5%) | 34 (85%) | 24 (60%) | 0.0429 * |
| 3 | 14 (17.5%) | 4 (10%) | 10 (25%) | |
| 8 (10%) | 2 (5%) | 6 (15%) | ||
| Number of embryos | ||||
| 1 | 44 (55%) | 20 (50%) | 24 (60%) | 0.1806 |
| 2 | 34 (42.5%) | 20 (50%) | 14 (60%) | |
| 3 | 2 (2.5%) | 0 | 2 (5%) | |
| Grade 4: high quality | 42 (52.50%) | 22 (55%) | 18 (45%) | 0.5026 |
| Grade 3: good quality | 38 (47.50%) | 18 (45%) | 22 (55%) | |
| Fresh Embryo | 14 (17.5) | 10 (25%) | 4 (10%) | 0.1395 |
| Frozen Embryo | 66 (82.5) | 30 (75%) | 36 (90%) | |
| IVF result: | ||||
| Successful | 8 (10%) | 6 (15%) | 2 (5%) | 0.2751 |
| Unsuccessful | 72 (90%) | 34 (85%) | 38 (80%) | |
| Successful pregnancy rate | 8 (10%) | 5 (12.5%) | 1 (2.5%) | 0.4643 |
| Abortion | 72 (90%) | 1 (2.5%) | 1 (2.5%) | |
| live birth rate | 5 (12.5%) | 5 (12.5%) | 0 | 0.0547 |
|
BMI: Body mass index. * Data presented as mean * Time interval from the first IVF. Grade 4: high quality Grade 3: good quality | ||||
The successful IVF process was observed in 6 (15%) and 2 (5%) patients among
the PRP
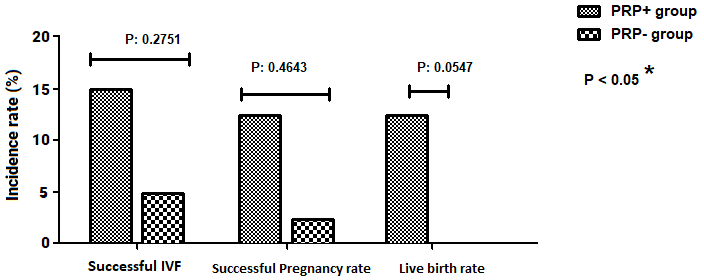 Fig. 2.
Fig. 2.Comparison of IVF-related variables (%) between PRP+ group and controls (PRP-group). Successful IVF: successful embryo implantation.
From a total of 8 women with successful IVF, 5 women were undergoing 2 embryos transfer and 3 women received single embryos. Also, from a total of 72 Unsuccessful IVF, 47 women have received 2 embryos and 25 were received single embryos. So, there was not any statistically significant correlation between successful IVF and the number of transferred embryos (P-Value: 0.223).
There was no azoospermia case or testicular sperm extraction (TESE) case.
The mean (SD) time elapsed since the first IVF was 1.66
In the control group, 39 cases reached the appropriate thickness, but one case did not reach the appropriate thickness as well as the successful pregnancy and live birth.
Receptive endometrium is one of several crucial factors for successful implantation and pregnancy [21]. The thin endometrium is not responsive to standard treatments, and it’s still a challenge for ART [1, 2]. However, immunological, hormonal, anatomical, and infectious factors along with known genetic factors are involved in 50% of Pregnancy loss cases; in this regard, about 9.8% of infertile couples have chromosomal balanced rearrangement [22].
Several studies have investigated the therapeutic effect of PRP on endometrial
growth and provided significant results [9, 10, 11, 12]. In this regard, Munson
et al.’s study evaluated the effects of PDGF on the proliferation of
endometrial epithelial cells. Their results showed the existence of functional
PDGF receptors on the endometrial epithelial cells that signal cell replication
[23]. Previously, Surrey and Halme (1991) demonstrated that PDGF secreted from
macrophages may have a significant role in endometrial cell proliferation [24].
The present study has investigated the effect of PRP containing PDGF on IVF
outcomes, whether the endometrial thickness is appropriate (7-10 mm) or not (
Based on our findings, the frequency of IVF success in the PRP
In fact, the mentioned studies have merely focused on the effect of PRP therapy
on the repairmen of endometrial thickness while some infertility causes aren’t
merely related to the endometrial thickness. In this sense, although our results
showed that PRP therapy can improve the IVF success rate whether the endometrial
thickness is appropriate or not, its improvement effect was not statistically
significant. So, our findings were inconsistent with the mentioned studies [12, 19]. Moreover, our study more comprehensively explored the effect of PRP therapy
on the improvement of the IVF process, pregnancy rate as well as live birth rate
following patient tracking. Although the successful pregnancy and live birth
rates were found to be higher in the PRP
Considering the relative similarity of the method between our study with other studies [12, 20], the low sample size seems to be a disruptive factor in achieving accurate results. Hence, it’s suggested to conduct more studies on a higher sample size to obtain more accurate results. However, our sample size was more than the sample size of previous studies.
In this clinical trial, the age range of 23-40 years old was considered; from a total of 8 women with successful IVF, 5 and 3 cases were under 35 and over 35 years old, respectively. Subsequently, from a total of 6 cases with a successful pregnancy, 3 cases were under 35 years old. Such a wide range of age may affect the patient’s prognosis and could be a selection bias.
The injection of PRP into the endometrium of IVF-failure patients may usefully affect the outcomes of the IVF process, pregnancy rate, and live birth rate, but not significantly so. Nevertheless, more researches are needed into a higher sample size to reach more accurate findings.
Considering a higher sample size for this study may give us more accurate findings.
Z. M designed the research study. Z. M and P. R performed the research. N. M provided help on the clinical step of the research. M.C. P provided help and advice on the PRP Preparation. Z. M and P. R analyzed the data and M.C. P helped in interpreting the results. Z. M wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the national research committee and with the 2008 Helsinki declaration and its later amendments or comparable ethical standards [Ethical Code: IR.AJUMS.REC.1396.634].
We would like to appreciate the Department of Obstetrics and Gynecology, fertility infertility and perinatology research center, Ahvaz Jundishapur University of medical sciences. And also Center for Reproductive Medicine Mahzyar.
This study was funded by Ahvaz Jundishapur University of Medical Sciences.
Dr. Mahvash zargar, First and correspondence author, has received research grants from Ahvaz Jundishapur University of Medical Sciences. Other coauthors report no conflicts of interest relevant to this article.
The authors declare no conflict of interest.
