Background: Previous studies had shown that major uterine wall
resection and reconstruction of the uterus (MURU) was safe and effective in the
treatment of adenomyosis. However, MURU results in loss of a significant amount
of myometrial and some endometrial tissues, which may have an impact on uterine
hemodynamics and ovarian function. Therefore, it is necessary to study the
changes of uterine hemodynamics and ovarian function in patients after MURU, in
order to provide an evidence-base for its clinical application.
Objective: To explore the effects of major uterine wall resection and
reconstruction of the uterus (MURU) on uterine hemodynamics and ovarian function
in patients with adenomyosis. Study design: The maximum
thickness of unilateral uterine muscle wall or the maximum diameters of
adenomyosis focus were measured by ultrasonography. Patients with adenomyosis
were divided into three groups: mild, moderate or severe. Fifty cases of
adenomyosis without fertility requirement were treated with MURU (observation
group), and levonorgestrel-releasing system (LNG-IUS) was placed in uterine
cavity during the operation. Fifty patients with intramural myoma of the uterus
were selected for hysteromyomectomy (control group). The changes of arterial
pulsation index (PI) and resistance index (RI) of uterine artery, as well as
antral follicle count (AFC) and serum antimullerian hormone (AMH) were observed
before operation and 1 month, 3 months, 6 months, 12 months and 18 months
post-operation. Results: No complications were observed in both
groups, no significant difference in uterine hemodynamics and ovarian function
were found between the two groups (P
Adenomyosis is a common gynecological disease, for which hysterectomy remains the main treatment. After a long-term study, the authors put forward “levonorgestrel intrauterine contraceptive system (LNG-IUS) three schemes” and “major uterine wall resection and reconstruction of the uterus (MURU)” to treat adenomyosis with different pathological stages. Our previous studies showed that MURU was safe and an effective procedure for patients with severe adenomyosis [1, 2]. Although MURU does not destroy the nutrient vessels of uterus and ovary, the removal of a large amount of uterine myometrium tissue by MURU may lead to post-operative changes in the internal anatomy of uterine myometrium and redistribution of blood flow, which may lead to changes in uterine and ovarian hemodynamics. Hemodynamic changes in either uterus or ovaries may have some impacts on ovarian function [3]. Therefore, it is necessary to make a comparative study of uterine hemodynamics and ovarian function before and after MURU, in order to clarify the effect of MURU on uterine hemodynamics and ovarian function, and to provide an evidence-base for the clinical application of MURU in women with adenomyosis.
From October 2014 to June 2017, patients with adenomyosis diagnosed based on the
Guideline for the Diagnosis and Treatment of Endometriosis [4] were admitted to
Zunyi Medical College and Women’s and Children’s Hospital of Zunyi Medical
College. Adenomyosis was classified into mild, moderate or severe according to
the maximum wall thickness (anterior wall, posterior wall or fundus) or maximum
diameter (MD) of adenomyosis focus measured by ultrasound [1]. Uterine volume is
calculated according to the approximate ellipse formula V =
Patients were included in the present study, if they had moderate to severe adenomyosis and those who were never treated by any other methods such as LNG-IUS or any surgery, had no contraindications for lower abdominal surgery, and had no fertility requirements.
Patients were excluded if they had mild adenomyosis, had fertility requirements, with adnexal tumors, history of adnexal surgery or contraindication for LNG-IUS use.
Fifty patients with moderate to severe adenomyosis who met the inclusion
criteria were set as observation group, of which 30 were moderate and 20 were
severe. Fifty patients with intramural myoma of uterus with surgical indications
were selected as control group. The diagnostic criteria of myoma were based on
the 8
(1) KAILIS30; SonoScape color doppler; frequency: 5.5-8.5 MHz.
(2) LNG-IUS (Mirena): Guangzhou Branch of Bayer Medical and Health Co., Ltd., 52 mg/branch.
(3) Human anti-Mullerian tube antibody (AMH Ab) ELISA kit: American R&D, detection range 0 ng/mL-300 ng/mL.
MURU was performed in the study population via the following steps (Fig. 1):
(1) Laparotomy under anesthesia (lower abdominal transverse incision was appropriate), temporarily blocking uterine blood supply with tourniquet in isthmus of uterus, separating first if there was adhesion.
(2) Longitudinal incision of the uterine muscular wall along the midline of the uterus to the bottom of the uterine cavity.
(3) The seromuscular layer was separated 5-10 mm below the serous to the isthmus level (external incision), 5-10 mm outside the endometrium, and the muscular layer was separated downward and converged with the external incision.
(4) Pruning redundant tissue (using electric knife as far as possible during operation, while effectively stopping bleeding and destroy the residual lesion tissue as far as possible). It was advisable to accommodate a LNG-IUS in uterine cavity.
(5) The seromuscular layers of the two sides were cut close to the midline, and the dead space was closed by intermittent suture to form a new uterus. Uterine reconstruction requirements: When suturing, the reconstructed uterus should be as close as possible to the normal uterus, and its volume is similar to or slightly larger than that of the normal uterus.
 Fig. 1.
Fig. 1.Standard surgical procedures of (major uterine wall resection and reconstruction of the uterus) MURU operation. (A) Longitudinal incision of muscular wall along or near the midline of uterine body; (B) Separation of bilateral muscular walls to isthmus level at about 0.5-1.0 cm below seromuscular layer; (C) Above the isthmus of uterus, the muscular wall within the seromuscular layer and 0.5 cm outside the mucosal layer has been resected; (D) LNG-IUS was implanted into the uterine cavity; (E) Uterine cavity was reconstructed by absorbable sutures; (F) Uterus was continuous suture and reconstructed.
Transabdominal or laparoscopic hysteromyomectomy was performed according to the size and number of myoma. All operations were performed according to standard procedures.
The uterine artery blood flow and antral follicle count were measured by color
doppler ultrasound at the same location by the same operator each time. 5 mL of
venous blood was extracted and centrifuged with a centrifuge (3000/min). The
upper serum was extracted and stored in a refrigerator at -70
The two groups were examined by color doppler pre-operatively and 1, 3, 6, 12 and 18 months post-operatively. The resistance index (RI) and pulsatility index (PI) of uterine artery were measured through uterine artery doppler. AFC and AMH were measured pre-operatively and 1, 3, 6, 12 and 18 months post-operatively in both groups.
Statistical analysis was conducted using SPSS 20.0 and data were expressed by
mean
MURU was performed in the patients of observation group via the following the
standard surgical procedures above (Fig. 1). There were no intraoperative or
postoperative complications between the observation and control groups. There was
no significant difference between the two groups in terms of age at diagnosis
(P = 0.841). The average age of the MURU group and the control group
were (40.5
The uterine artery was well displayed in both groups pre- and post-operatively,
and spectrum doppler showed high velocity and high resistance type with uterine
artery (Fig. 2,3). There was no significant difference in PI and RI
between the two groups before and after MURU (P
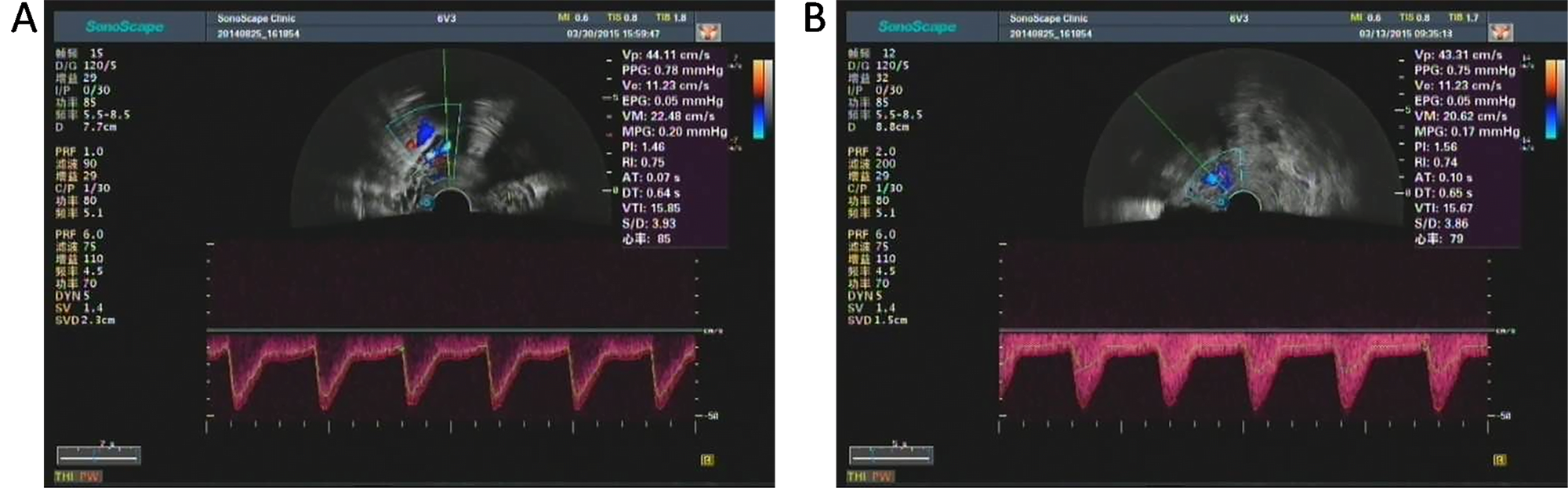 Fig. 2.
Fig. 2.Color doppler spectrogram and hemodynamic indexes of uterine artery of one patient in control group before (A) and after (B) hysteromyomectomy. The PI and RI of preoperative patient were 1.46 and 0.75, respectively. The PI and RI of postoperative patient were 1.56 and 0.74, respectively. Both the PI and RI showed no significant difference before and after hysteromyomectomy.
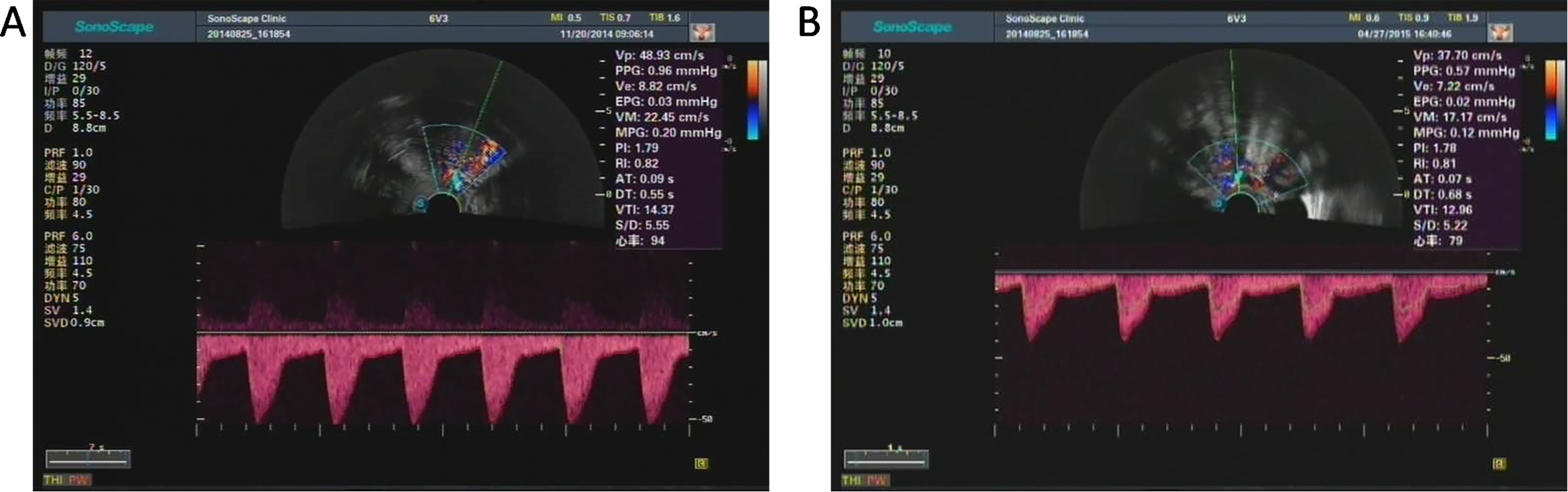 Fig. 3.
Fig. 3.Color doppler spectrogram and hemodynamic indexes of uterine artery of one patient in observation group before (A) and after (B) MURU. The PI and RI of preoperative patient were 1.79 and 0.82, respectively. The PI and RI of postoperative patient were 1.78 and 0.81, respectively. Both the PI and RI showed no significant difference before and after MURU.
| Index | Pre | 1 month | 3 month | 6 month | 12 month | 18 month | |
| operative | postoperative | postoperative | postoperative | postoperative | postoperative | ||
| PI | Control group | 1.874 |
1.868 |
1.896 |
1.885 |
1.850 |
1.870 |
| MURU group | 1.887 |
1.910 |
1.930 |
1.905 |
1.912 |
1.926 | |
| RI | Control group | 0.810 |
0.813 |
0.820 |
0.820 |
0.820 |
0.813 |
| MURU group | 0.810 |
0.815 |
0.820 |
0.816 |
0.822 |
0.817 | |
| Compared with preoperative group, P | |||||||
As shown in Table 2, there was no significant difference in AFC and AMH between
the two groups at each time point (P
| Index | Pre | 1 month | 3 months | 6 months | 12 months | 18 months | |
| operative | postoperative | postoperative | postoperative | postoperative | postoperative | ||
| AFC | Control group | 6.360 |
6.280 |
6.260 |
6.340 |
6.380 |
6.360 |
| MURU group | 6.340 |
6.360 |
6.280 |
6.420 |
6.340 |
6.340 | |
| AMH | Control group | 1.516 |
1.534 |
1.520 |
1.529 |
1.533 |
1.509 |
| MURU group | 1.517 |
1.495 |
1.508 |
1.524 |
1.524 |
1.502 | |
| Compared with preoperative group, P | |||||||
Adenomyosis is a common and frequent gynecological disease. In recent years, the prevalence of adenomyosis always stayed at high level, although there were differences in diagnostic criteria, sampling methods and observer bias [5]. There are many methods to treat adenomyosis, which can be divided into conservative treatments and radical treatments [6]. Conservative treatments, such as drug therapy, physiotherapy and local lesion resection, but they all have many problems such as great difference in curative effect, large side effects and high recurrence rate [7]. Hysterectomy has long been the most important treatment for severe patients and those who failed after conservative treatment. The significant physiological and psychological effects of hysterectomy on women should not be under-estimated [8], therefore, uterine preservation is a definite option that must be considered for patients with adenomyosis, where possible. Our previous studies showed that MURU was safe and effective in the treatment of moderate to severe adenomyosis, therefore hysterectomymay be avoided for these patients [1, 2].
Other non-hysterectomy treatment options have been suggested for adenomyosis; such as high intensity focused ultrasound (HIFU) and uterine artery embolization (UAE). HIFU is a non-invasive and conservative treatment for adenomyosis, and it offers many benefits which include few complications during pregnancy [9]. However, there is still a lack of large-scale randomized controlled study to further compare the effect of HIFU with other in adenomyosis patients. On the other hand, uterine artery embolization (UAE) is another alternative to hysterectomy for symptomatic adenomyosis [10], but its impacts on ovarian function and endometrium cannot be ignored. The negative impact of UAE on ovarian function is mainly caused by the reverse flow of embolic agent into ovarian artery [11]. Both ovarian dysfunction and uterine artery embolization may lead to endometrial necrosis, which will bring about decreased menstrual volume and amenorrhea, especially in women older than 45 years [10]. As there were few reports about pregnancy outcome after UAE treatment and no definite conclusion on its effects on reproductive health [12]. Therefore, UAE may not be suitable for adenomyosis patients who wish to retain fertility.
Transvaginal color doppler (TVCD) can be used to observe the changes of uterine
vascular morphology and hemodynamics [13]. The commonly used indicators are PSV,
EDV, RI, PI and so on. PI is the index of vascular compliance and elasticity, RI
is the index of vascular contraction and resistance. RI and PI can reflect the
distal resistance and the elasticity of artery wall in a certain range, and can
eliminate the influence of blood flow angle [14]. Therefore, PI and RI can more
accurately reflect the distal resistance, the elasticity of artery wall and the
flow perfusion of the measured organs. Uterine artery spectrum doppler before and
after MURU treatment showed a high-speed and high-resistance type. It can be
concluded that there was no significant effect on uterine hemodynamics in
adenomyosis patients treated with MURU. The above results showed that there were
no significant changes in uterine hemodynamics parameters (RI, PI) between the
MURU and control groups (P
Large part of uterine myometrium tissues were resected after MURU. The significant reduce of uterine volume will lead to the redistribution of uterine myometrium blood flow and reduce the demand for uterine blood flow [3]. In this case, the uterine branch of uterine artery (the main supplying vessel of uterine body) will inevitably shrink “adaptively”. However, this reduction was not caused by the blood vessel itself (MURU did not involve the blood vessels on both sides of the uterus), and the compliance and elasticity of the blood vessel itself would not be affected, therefore, MURU did not lead to PI changes.
On the other hand, MURU resection of all myometrial tissues above isthmus, 5-10 mm in seromuscular layer and 5-10 mm outside mucosal layer. It will cause the destruction of blood vessels in uterine muscular wall and affect the blood perfusion and supply of uterus. However, it could be inferred that this process should be very short, the reconstructed uterus could obtain blood supply through collateral circulation and vascular regeneration rapidly, and otherwise it will lead to uterine ischemia and necrosis. Therefore, although MURU may change uterine blood perfusion (RI) in a short time after operation, it will not affect the RI of uterus reconstruction significantly due to good collateral circulation, vasculogenesis and similar tissue properties before and after operation.
A decrease in menstrual volume or amenorrhea was observed in most of the patients who combined MURU with LNG-IUS. However, these patients did not appear to experience hot flushes, night sweats, insomnia, anxiety and other perimenopausal symptoms. In addition, studies have shown that hysteromyomectomy does not affect the blood supply of ovaries [15], and has less impact on accessories, so the short-term and long-term impact on ovarian function is not obvious. Our results also showed that AFC and AMH did not change significantly in this group within 18 months after operation, that is, ovarian function was not significantly affected. Therefore, the main reason for the decrease of menstrual volume in this group was related to both LNG-IUS and the removal of part of the endometrium by MURU.
After the placement of LNG-IUS into uterus, levonorgestrel can maintain a high concentration of levonorgestrel in the endometrium and continue to function, fundamentally changing the pathological state of the endometrium [16]. These modified endometrium, even if heterotopic endometrium will not cause endometriosis or adenomyosis [17]. Therefore, LNG-IUS has a good effect in preventing the recurrence of pelvic pain, ectopic lesions and adenomyosis. Studies had shown that the placement of LNG-IUS in uterus will not affect uterine blood flow and ovarian function, but will lead to a significant reduction in menstrual volume, or even amenorrhoea [18, 19], which can well explain the changes in menstrual volume in the patients who combined MURU with LNG-IUS.
MURU is a new method for the treatment of adenomyosis. Although MURU removed a large amount of myometrial tissues and some endometrial tissues, the procedure itself did not destroy the nutrient vessels of the uterus and ovary, and also did not cause direct physical damage to the ovary. Although MURU changed the internal anatomical structure of the uterus, resulting in redistribution of blood flow, this change did not affect ovarian blood supply. Therefore, it would not have a significant negative impact on ovarian function [20].
MURU reconstructs uterine wall by suturing musculoserosal tissue in one layer, rather than using double or triple flap method. This might increase the risk of uterine rupture during pregnancy. Thus, MURU is a procedure that will benefit women who have no fertility requirements. In the near future, we will deeply continue to explore surgical methods that can be used for women with fertility requirements, and will strive to make MURU surgery benefit more women.
In conclusion, MURU operation did not have significant negative impact on uterine hemodynamics and ovarian function during the observation period in this study, but its long-term impact still needs to be further reviewed and observed in larger studies.
X.Y. conceived and designed the experiments; X.Y., M.M., G.H., L.H., Y.M. and L.Z. performed the experiments; L.Z. and H.L. analyzed the data; X.Y. and H.L. wrote the paper.
This study was approved by the Ethics Committee of Zunyi Medical College, No. Lun Shen (2014) 1-046. All patients signed informed consent.
This work was funded by National Natural Science Foundation Project of China (NSFC) (NO.81460233, 81660293).
The authors declare no conflict of interest.
