Academic Editor: Mahmoud S. Ahmed
Background: Chicken Ovalbumin Upstream Promoter-Transcription Factor I
(COUP-TFI) is a member of the steroid/thyroid nuclear receptor
superfamily. The aim of this study was to investigate whether absence of this
gene affects placental development and fetal growth in a COUP-TFI
knockout mouse model. Methods: Placentas of COUP-TFI-knockout
(COUP-TFI KO) and wild-type (WT) were collected at 18.5 days
post-coitum. The expression level of the following genes known to be involved in
different key molecular pathways was evaluated: BCL2 Associated X (Bax)
and B-cell lymphoma 2 (Bcl-2) (apoptosis), p21, p53
and
At the basis of the development of a viable pregnancy in mammals there is the adhesion and implantation of a blastocyst to the endometrial epithelium. While the inner cell mass of the blastocyst will form the embryo, the outer layer, called the trophoblast, further develops into the maternal decidua and gives rise to the placenta. This latter represents the pivotal organ linking the developing embryo to the mother, providing the necessary oxygen supply and nutrient exchange [1]. Proliferation, apoptosis, and angiogenesis are crucial mechanisms involved in the correct development and remodeling of all the complex structures that make up the placenta. Impairments of this processes can result in several complications, including a deficient growth of ongoing pregnancy [2].
Chicken Ovalbumin Upstream Promoter-Transcription Factor I (COUP-TFI), also known as nuclear receptor subfamily 2, group F, member 1 (NR2F1) is an orphan nuclear receptor factor and member of the steroid/thyroid hormone receptor superfamily, mainly expressed in the central and peripheral nervous systems. The mammalian COUP-TFI plays a key role during metabolic homeostasis as well as organogenesis through cell fate determination, differentiation, proliferation, and apoptosis [3, 4]. Overall, the high conservation of amino acid sequence between species suggests vital evolutionary conserved functions worth being explored, also when considering the process of placental development [5, 6, 7].
Earlier studies conducted on human placenta highlight the potential key role played by COUP-TFI. An in-silico investigation of transcriptional profile obtained using a microarray approach revealed that COUP-TFI was highly associated with self-renewal and differentiation of human chorionic trophoblast progenitor cells [8]. Furthermore, COUP-TFI was indicated in a meta-analysis among the genes that seems to play a role in the development of pre-eclampsia, a complication of placental function often related to fetal growth restriction [9]. However, whether and how COUP-TFI could influence the process of placental development has not been evaluated yet.
Compared to the monolayer in human placenta (hemomonochorial), placenta in mice is composed by three trophoblast layers (hemotrichorial) [10]. At embryological (E) day 10.5, when mid-gestation begins, all the layers of the placenta are formed, including the outermost maternal part, called the decidua, and the fetal part with the triple trophoblastic layer [11, 12]. Embryonic development ends with the mid-gestation phase at E13.5, thereafter the fetus matures until the time of birth, around day E19.5 [13].
Transgenic COUP-TFI KO mice have been developed to shed more light into COUP-TFI functions [14]. Upon COUP-TFI loss, mice litter show a high incidence of perinatal mortality due to several neuronal malformations, particularly in the glossopharyngeal ganglion, defects in axonal arborization, and loss of cortical patterning due to the absence of thalamocortical connections [7, 15].
The aim of this study was to explore whether and how COUP-TFI deletion in mice could interfere with placental development in terms of expression of some genes related to proliferation, apoptosis, and angiogenesis and in terms of neonatal weight at birth.
COUP-TFI KO (COUP-TFI –/–) mice were generated and subsequently
genotyped using the following primers, as previously described: forward
5
All samples were kept on ice during dissection, then quickly transferred into
500
The following genes have been included as markers for placental development:
hypoxia-inducible factor 1-alpha (Hif1
| Primer | Name |
| Sense 5′- CCGAGAATGGGAAGCTTGTC -3′ | Gapdh |
| Antisense 5′-TCTCGTGGTTCACACCCATC -3′ | Gapdh |
| Sense 5′- CCTTTTTGCTACAGGGTTTCATC -3′ | BAX |
| Antisense 5′-AGCTCCATATTGCTGTCCAGTT -3′ | BAX |
| Sense 5′- AAGCTGTCACAGAGGGGCTA -3′ | Bcl-2 |
| Antisense 5′-TCAGGCTGGAAGGAGAAGATG -3′ | Bcl-2 |
| Sense 5′- TGTCGCTGTCTTGCACTCTG -3′ | p21 |
| Antisense 5′-CCAATCTGCGCTTGGAGTGATA -3′ | p21 |
| Sense 5′- TGCTCACCCTGGCTAAAGTT -3′ | p53 |
| Antisense 5′-GTCCATGCAGTGAGGTGATG -3′ | p53 |
| Sense 5′- ATGAACTTTCTGCTCTCTTGGGT -3′ | VEGF-A |
| Antisense 5′-CACAGGACGGCTTGAAGATGTA -3′ | VEGF-A |
| Sense 5′- TGCTGGTCATGAAGCTGTTC -3′ | PlGF |
| Antisense 5′-GGACACAGGACGGACTGAAT -3′ | PlGF |
| Sense 5′- GACGATGAACATCAAGTCAGCA -3′ | HIF1 |
| Antisense 5′-GGAATGGGTTCACAAATCAGCAC -3′ | HIF1 |
| Sense 5′- GAGGAGGATGAGGGTGTCTATAG -3′ | Flt-1 |
| Antisense 5′-TGATCAGCTCCAGGTTTGACT -3′ | Flt-1 |
| Sense 5′- CTTCCAAGGACAGCCAAGAGT -3′ | ENG |
| Antisense 5′-GTGGTTGCCATTCAAGTGTGG -3′ | ENG |
| Sense 5′- TCGAAGACATGCCGTTGGG -3′ | INHA |
| Antisense 5′-AGCTGGCTGGTCCTCACA -3′ | INHA |
The performed reactions were run in triplicate in three independent experiments.
The mRNA quantification was expressed in terms of the cycle threshold
(Ct). From each triplicate run, the means of the Ct values were calculated and
used for further analysis. All gene expression levels were normalized on the
values of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase
(GAPDH). Differences between the Ct values of the tested genes and those
of the reference gene were calculated as
Data were analyzed using R v3.5.3 with p
According to RT-qPCR data, the most highly expressed gene in both
COUP-TFI KO and WT placental tissue was
HIF1
 Fig. 1.
Fig. 1.Key placental genes expression pattern. Box plot showing fold
change values (2
| COUP-TFI KO | Wilde type (WT) | p | |
| BAX | 0.035 (0.033–0.036) | 0.020 (0.020–0.021) | |
| Bcl-2 | 0.003 (0.002–0.003) | 0.003 (0.002–0.005) | 0.841 |
| p21 | 0.031 (0.023–0.047) | 0.020 (0.017–0.051) | 1.000 |
| p53 | 0.001 (0.000–0.001) | 0.000 (0.000–0.001) | 0.690 |
| ENG | 0.004 (0.004–0.010) | 0.008 (0.003–0.011) | 1.000 |
| HIF1 |
0.047 (0.045–0.071) | 0.061 (0.037–0.073) | 1.000 |
| Flt1 | 0.009 (0.007–0.018) | 0.010 (0.005–0.010) | 1.000 |
| PlGF | 0.015 (0.011–0.020) | 0.008 (0.004–0.030) | 0.548 |
| VEGF1 | 0.010 (0.006–0.011) | 0.003 (0.002–0.005) | |
| INHA | 0.003 (0.001–0.003) | 0.001 (0.001–0.003) | 1.000 |
Correlations between all evaluated transcripts in WT and
COUP-TFI KO mice are shown in Fig. 2. We found significant positive
correlations in the placental tissue of WT mice between the following mRNA pairs:
Bcl-2 and INHA (rho = 1 and p
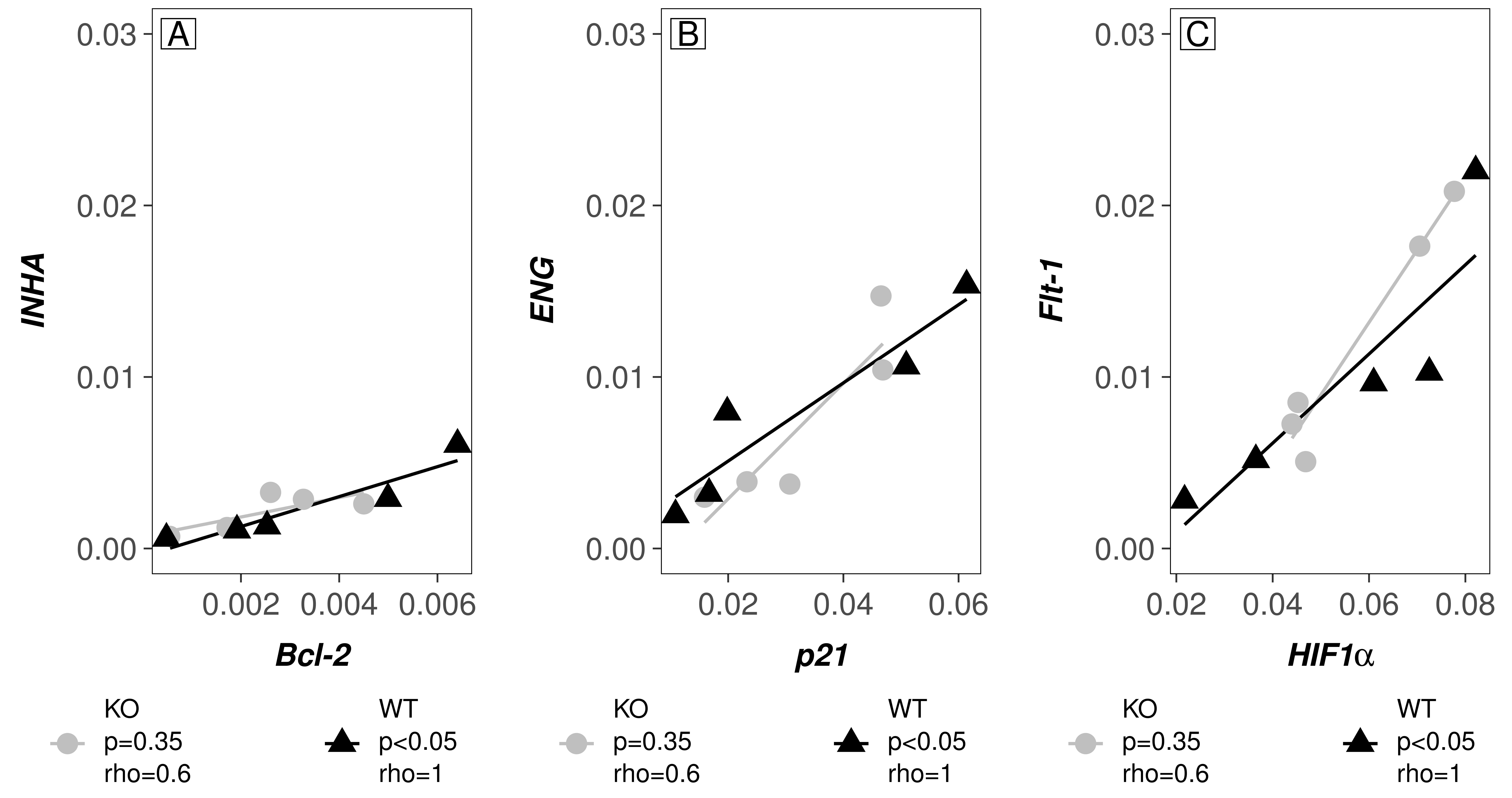 Fig. 2.
Fig. 2.Plots showing correlations between placental genes in
WT and COUP-TFI KO mice. Panel (A) Shows correlation between
INHA and Bcl-2. Panel (B) Shows correlation between ENG and
p21. And Panel (C) Shows correlation between HIF1
Finally, we found a reduction of weight in COUP-TFI KO pups
compared to littermates controls. The mean weight of WT mice was 1.6 grams
(
In this study, we assessed the role of mouse COUP-TFI in regulating the
expression of major genes involved in placentation. We found that VEGF-A
and Bax mRNA expression were increased in COUP-TFI KO compared
to wild-type mouse placentas, suggesting an impairment of apoptotic and
angiogenetic pathways in mutant placental tissue. The positive correlations
observed in normal placental tissue between Bcl-2 and INHA,
p21 and ENG, and HIF1
We focused our attention on COUP-TFI because this family of nuclear
receptors carries out vital roles in physiological processes, including
proliferation, apoptosis and cell signaling [15, 20]. Thanks to the level of
evolutionary conservation of COUP-TFI, understanding the pathological
pathway in relation to its expression in mouse models could help to better focus
future studies on genes known to be relevant during the process of physiological
placental development. In particular, we analyzed the expression of
HIF1
When considering the influence of COUP-TFI on the expression of the
most common angiogenetic factors, we found that VEGF-A mRNA was
consistently up-regulated in COUP-TFI KO placentas compared to control
mice. The isoform A of the vascular endothelial growth factor (VEGF-A),
belonging to the VEGF family, is considered the most crucial factor promoting the
differentiation of mesenchymal cells in villi into hemangioblastic stem cells.
VEGF-A expression is induced by hypoxia, as a potent stimulus, and is
mediated via HIF1
Supporting this evidence, we found in COUP-TFI mutants a loss in the
positive correlation between mRNA expression of HIF1
Considering the most common genes involved in cell proliferation and survival control, we observed an increase of Bax mRNA in mutant placentas. An augmented Bax expression is in line with other studies conducted on human placenta [33, 34, 35]. Bax is a pro-apoptotic protein that exerts, in concert with the anti-apoptotic protein Bcl-2, a crucial role in apoptosis. Both are regulated by the p53 tumor suppressor gene [36]. Apoptosis contributes to the turnover of villous trophoblasts and plays a crucial function in the remodeling of spiral arteries in human placenta. Apoptosis in placental villi changes throughout normal pregnancy: it is low in the first trimester, increases in the second, and then reaches the highest levels beyond 40 weeks of gestation [37]. Furthermore, the amount of apoptosis is increased in villous trophoblast in placental pathologies, including preeclampsia [38].
In addition, we observed a significant positive correlation of Bcl-2 and INHA in normal mice. This could be due to a regulatory role of on trophoblast growth through inhibition of the activin receptor, known to have a fundamental role in trophoblast development and correct placentation [39]. This, in turn, could result in reduced placenta proliferation and increased apoptosis characterizing old placentas at the end of gestation [40]. According to our study, this correlation seems to be altered by the absence of COUP-TFI.
Interestingly, we also observed a lower level of p53 expression than its downstream target p21 in COUP-TFI KO samples, and a positive correlation of the expression rate of the two genes. These data confirmed previous results on human placentas [21]. We can further hypothesize a role of p21 independent of p53. Usually p53, through p21, promotes cell cycle arrest or apoptosis via the augmented expression of Bax [36, 41]. In the current study, we found a significant positive correlation between p21 and ENG transcripts. ENG is part of the transforming growth factor-beta receptor complex. Angiogenesis, apoptosis, and cell cycle arrest could be promoted by the transforming growth factor-beta pathway, reported to be implicated in fetal growth restriction [42]. p21 could also possibly interact in placental tissue with this cascade triggered by the transforming growth factor-beta receptor complex [43]. As the correlation between p53 and p21 is lost in mutant mice, this interaction could be disturbed by the absence of COUP-TFI.
Finally, we looked at the link of the mouse phenotype to placental function in terms of weight of pups recorded at birth. Interestingly, our results showed that COUP-TFI KO pups presented a significant lower weight than WT littermate controls. These data further support the pathological significance of COUP-TFI in placental development, potentially related to fetal growth restriction, a common complication associated with impaired placental function in humans [9, 44, 45]. Our preliminary results invite further studies on specific downstream cascades of molecular markers linked to COUP-TFI, both in mouse models and humans.
This explorative study aimed to identify potential markers involved in impaired placental function linked to COUP-TFI loss-of-function but lacks a detailed analysis of mechanisms underlying the downstream regulation of angiogenic, cell regulation and apoptotic factors included in this mouse model. Moreover, in our experiments we have not assessed the function COUP transcription factor 2 (COUP-TFII), a homolog to COUP-TFI sometimes compensating COUP-TFI functions. COUP-TFI and COUP-TFII expression patterns overlaps in many regions and organs, possibly resulting in redundant functions [6, 46, 47]. Thus, COUP-TFII may be able to compensate for the absence of COUP-TFI in COUP-TFI KO mice. Further experiments on placentas lacking both COUP-TF members derive from crossing between COUP-TFI and COUP-TFII mutant mice, could shed new light on the interplay between these nuclear receptors during placental development. Exploring in more detail placental morphology could also be a topic of future interest.
The present study provides evidence that the absence of COUP-TFI influences the expression levels of two key effectors of mouse placental angiogenesis and apoptosis, VEGF-A and Bax. Consistently, we showed that COUP-TFI KO mice presented a significant lower weight at delivery than WT littermate controls. Hence, we propose that COUP-TFI plays an important role for placental development and function, even though further studies will be necessary to dissect the molecular dynamics governing a COUP-TFI-dependent placenta development.
Bax, BCL2-associated X protein; Bcl-2, B-cell lymphoma 2;
COUP-TFI, Chicken Ovalbumin Upstream Promoter-Transcription Factor I;
COUP-TFII, Chicken Ovalbumin Upstream Promoter-Transcription Factor II;
ENG, Endoglin; FLT-1, Fms-like tyrosine kinase 1 (also known as
VEGFR1); HIF1
LV, SM, APL, MS, LM, AF—substantial contributions to conception and design. LV, SM, MB, APL, MO, SB, MS, LM, AF—substantial contributions to acquisition of data or to analysis and interpretation of data. LV, SM, MB, APL, MO, SB, LD, CDL, MS, LM, AF—drafting the article or revising it critically for important intellectual content. All authors read and approved the final manuscript.
All mouse experiments were conducted in accordance with the relevant national and international guidelines and regulations (European Union rules; 2010/63/UE), and with approval by the local ethical committee in France (CIEPAL NCE/2019-548).
The authors would like to thank Christian Alfano from iBV, Nice, France, for initial interest in this study and the Pathology of the AOU Santa Maria della Misericordia, University of Udine, Italy, who collaborated in the study.
This research received no external funding.
The authors declare no conflict of interest. APL is the Editor of this journal, given his role as Editor, had no involvement in the peer-review of this article and has no access to information regarding its peer-review.
The data that support the findings of this study are available, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Internal Review Board.
