Academic Editor: Shikun He
Objective: To evaluate the effect of 0.2% ambroxol eye drop on
tear secretion and corneal healing on a rabbit dry eye model, and to delineate
potential underlying mechanisms. Materials and method: A mixed
mechanism dry eye model was created using 12 healthy New Zealand rabbits
by excision of the main lacrimal glands, Harderian gland and nictitating
membrane. Establishment of the model was confirmed by the decrease of Schirmer I
and increase of corneal fluorescein staining scores. Two weeks after model
creation, the rabbits were randomly and evenly divided into NaCl, 0.1% sodium
hyaluronate and 0.2% ambroxol groups. Each group was administered the respective
eye drops 4 times a day for four weeks. The Schirmer I test and corneal
fluorescein staining were performed at two and four weeks. After four weeks of
treatment, the animals were sacrificed and the conjunctiva and eyelid specimens
collected. Inflammatory factors IL-8, TNF-
Altered tear film (TF) composition is a common cause of dry eye disease (DED) which can further lead to ocular surface inflammation and loss of tear homeostasis. There are two types of DED in current classification— aqueous-deficient and evaporative, related directly to decrease in aqueous or lipid components of TF [1]. Evaporative DED is more common than aqueous-deficient type, but more than one-third of patients will have a combination of both. DED represents one of the biggest unmet needs in ophthalmology. Current methods can often alleviate but not cure DED. Therefore, in-depth study of the pathogenesis of DED, as well as the discovery of new drugs are still vitally important.
The natural hydrophobicity of cell membrane lipids requires mucin to fix the tear film on the ocular surface for lubrication and protection. Our early study found that transconjunctival fluid transport driven by osmotic pressure gradient is mainly carried out through aquaporin, and its increased expression can drive the expression and secretion of MUC5AC, the most important gel-forming mucin in tears, from conjunctival epithelial goblet cells [2]. We have also recently discovered that ambroxol can stimulate the expression of aquaporin 5 (AQP5) and mucin 5AC (MUC5AC) in vitro, and can promote tear secretion in normal rabbits [3], demonstrating potential as a drug for DED. Ambroxol is a secretolytic and mucoactive agent primarily used to treat respiratory diseases associated with viscid mucus [4]. Ambroxol is reported as having multiple clinically relevant actions including reduction of mucus viscosity, stimulation of airway surfactant secretion [5], anti-inflammation [6] and analgesia [7]. All these pharmaceutical properties are of potential benefit for DED.
To date, various lacrimal deficiency dry eye animal models have been employed in studies such as by inducing autoimmune reaction, removing lacrimal gland, blocking nerve, and using parasympathetic nerve inhibitor [8, 9, 10], all with pros and cons. In this study, we chose a mixed-mechanism rabbit dry eye model reliably used by our group [11], and used NaCl and sodium hyaluronate eye drops as controls, to evaluate the effect of 0.2% ambroxol eye drop on dry eye. We also attempted to explore other potential mechanisms of action by ambroxol in this dry eye model.
A total of 12 male New Zealand white rabbits (24 eyes, Xinhua Research Animal
Farm, Guangzhou, Guangdong, China) weighing 2.0–2.5 kg were purchased. All
animals had passed quarantine inspection and been given one week adaptation time
to the new environment prior to enrollment into the study. Corneal fluorescein
staining score was recorded and Schirmer I test conducted in each eye as baseline
prior to surgery. The animals were reared at the laboratory of Shenzhen Lingxian
Medical Service Co., Ltd., under standard conditions (22
The main lacrimal gland, Harderian gland (HG) and nictitating membrane (NM) were excised in both eyes as per previous publications [12, 13] with minor modification. Identical procedure was performed on both eyes. Briefly, under general anesthesia, the skin of both periocular regions was shaved, disinfected with 5% betadine and draped. The orbital and palpebral lobes of the superior lacrimal gland was first removed (Fig. 1a,b,c,f) as described previously [13]. Subsequently, the inferior lobe was removed through a linear skin incision along the inferior and lateral orbital rims (Fig. 1d,f). The NM was then snipped off at its base, the HG extracted through the same wound and removed in its entirety (Fig. 1e,f). Hemostasis was achieved by gentle tamponade. The orbit septum and skin wound were then closed separately with interrupted 5-0 vicryl sutures. Ofloxacin ophthalmic ointment (Santen Pharmaceutical Co., Ltd., Osaka, Japan) was administered topically at the surgical wounds twice a day for 7 days for infection prophylaxis.
 Fig. 1.
Fig. 1.Surgical steps involved in the creation of a mixed-mechanism rabbit dry eye model. (a) Superior orbital incision. (b) Extraction of the superaorbital lacrimal gland. (c) Identification of the palpebral lacrimal gland for excision. (d) Extraction of the infraorbital lacrimal gland. (e) Excision of the nictitating membrane and Harderian gland. (f) Tissues removed (from left to right) are the supraorbital lacrimal gland, infraorbital lacrimal gland, palpebral lacrimal gland, nictitating membrane, and Harderian gland.
All animals were evaluated prior to model creation surgeries and 2 weeks after for confirmation of the establishement of the dry eye medol. After 2 weeks, the 12 animals were randomly divided into 3 groups: 0.9% NaCl, 0.1% sodium hyaluronate (Lianbang Pharmatheutical Ltd, Corp, Zhuhai, China, Lot#: M0861201-N02) and 0.2% ambroxol (MedChem Express, Shanghai, USA) groups. Each group had 4 rabbits (8 eyes). Each eye then received the respective eye drop 4 times a day, starting from week 2, and was evaluated every 2 weeks for additional of 4 weeks.
The fluorescein sodium staining test paper was first wetted with one drop of normal saline and then the tip of paper gently touched the lower conjunctival sac of each rabbit eye. After several natural blinking, each rabbit eye was observed under the slit lamp with diffuse cobalt blue light and the staining of cornea recorded by photographs. Using the standard NEI15 method, the cornea was divided into five zones: central, upper, lower, temporal and nasal. Each zone was scored according to severity and pattern of staining: 0 for no staining, 1 for scattered punctate staining, 2 for diffuse punctate staining, 3 for filamentous, blot or patchy staining, with a total possible score of 15 points in each eye.
A standard 5
At the end of the study, the animals were sacrificed by air embolism, and the
bulbar conjunctival tissue near the superior fornix and a segment of
full-thickness superior lid tissue were removed from each rabbit eye. The bulbar
conjunctival tissue specimens were blotted with filter paper first and weighed.
PBS was then added to the tissue at a 9:1 ratio (e.g., 9 mL PBS to 1 g specimen).
The conjunctival specimens were homogenized in PBS and placed at –20
Rabbit upper lid tissue sections were deparaffinized and rehydrated first, washed with double distilled water, and then stained with oil red working solution for 10–15 minutes according to manufacture recommended protocol (Shanghai Yuanye Bio-Technology Co., Ltd., Shanghai, China). Last, the sections were counter-stained with hematoxylin for observation under light microscope. The oil content in the tissue appeared bright red, and the cell nucleus blue.
After centrifugation, the supernatants of bulbar conjunctival homogenates were
taken for quantitative assessment of IL-8, TNF-
GraphPad Prism 8.3.0 software (GraphPad Software, LLC. San Diego, CA, USA) was used for statistical analysis. Statistical significances among Schirmer I values, corneal fluorescein staining scores, cytokine and MUC5AC concentrations before and after ambroxol treatment were first analyzed by one-way Anova, and in each group, further compared by Fishers LSD test.
All 12 rabbits suffered no complications from surgeries and appeared well
physically throughout the study. Compared with the baseline, the values of
Schirmer I at two weeks after model creation had decreased significantly (20.35
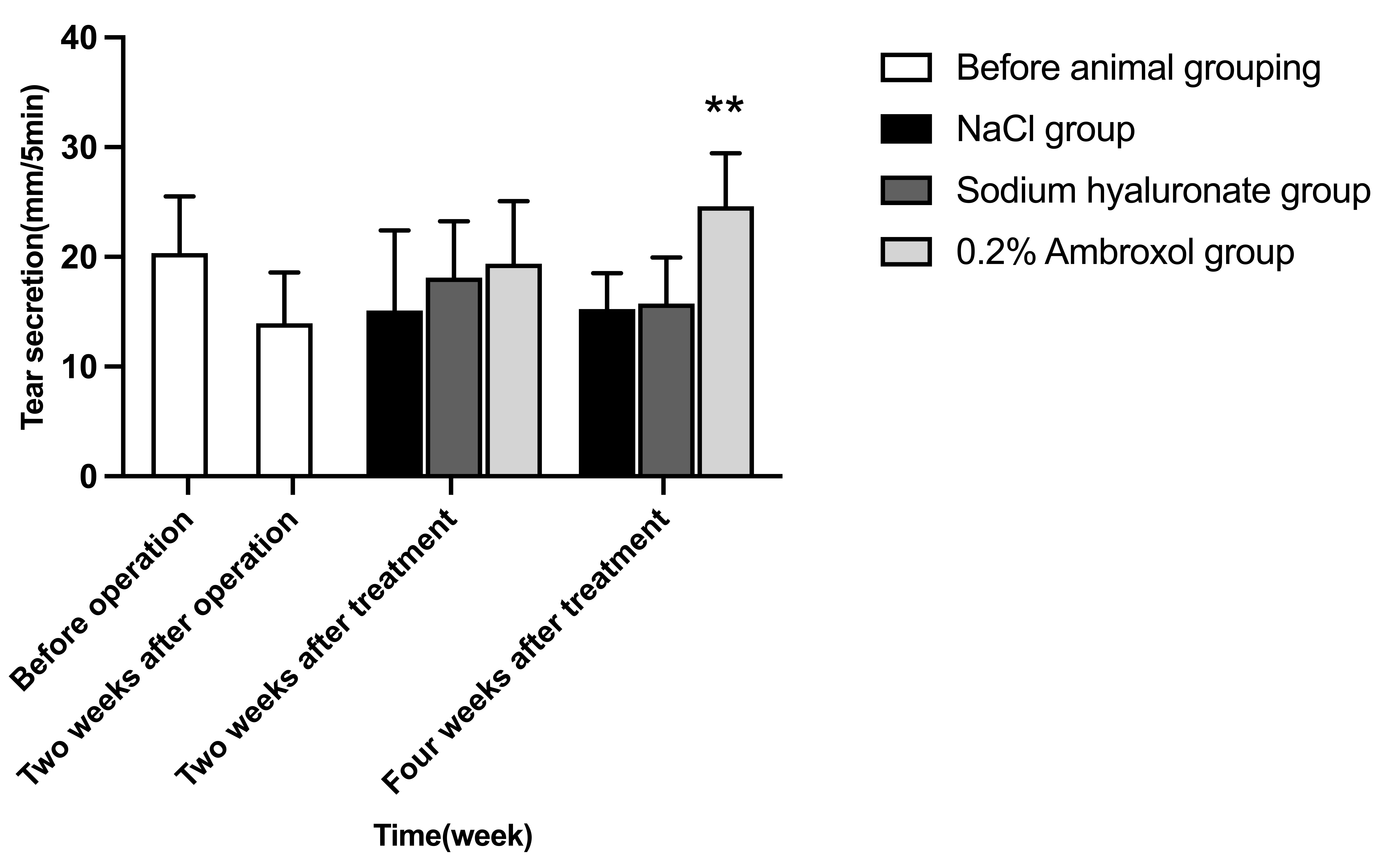 Fig. 2.
Fig. 2.Comparison of tear secretion among groups at two weeks after
operation, two and four weeks after treatment. Comparison of ambroxol group with
NaCl group, **p
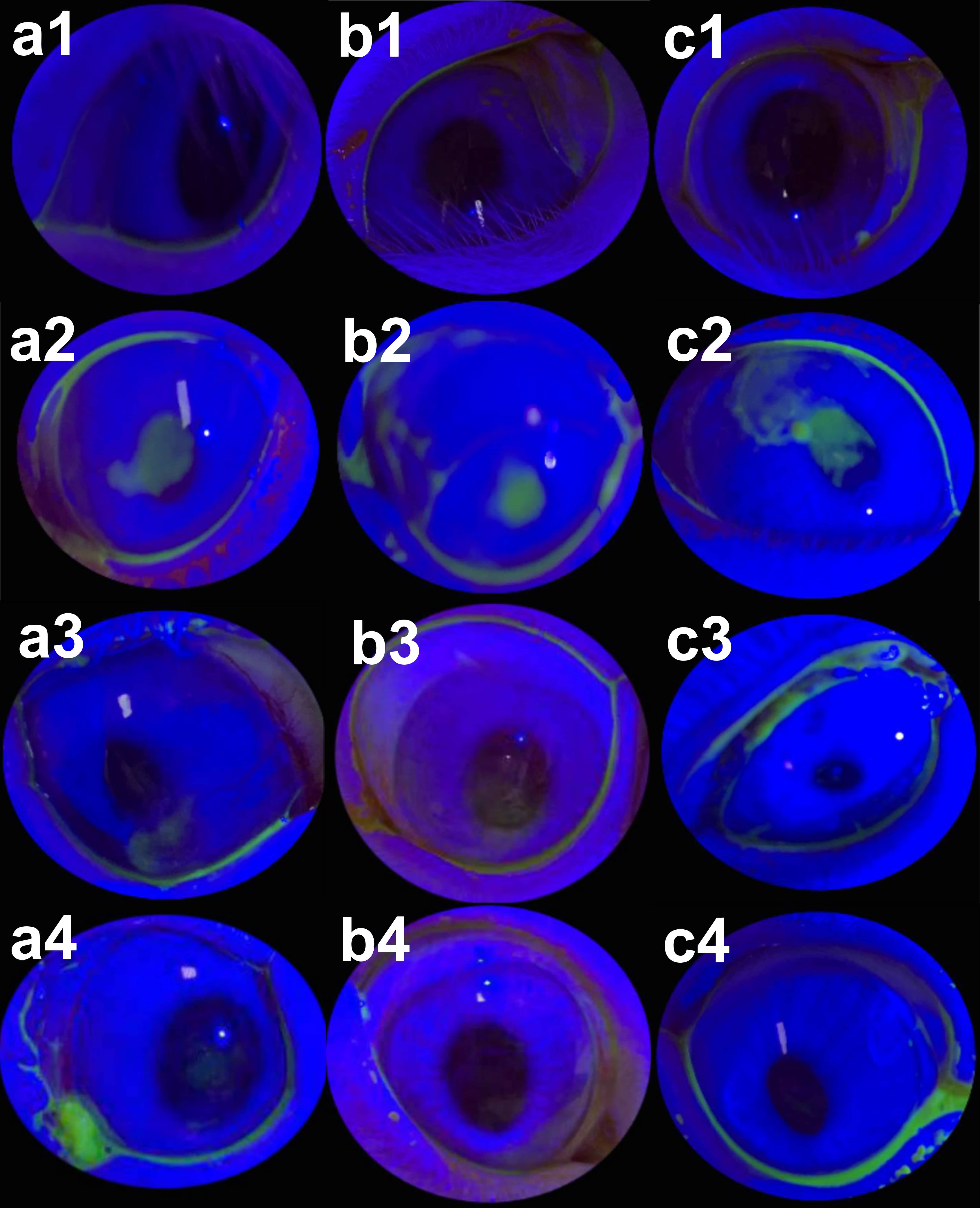 Fig. 3.
Fig. 3.Results of corneal fluorescein staining before operation (1), two weeks after operation (2), two (3) and four weeks (4) after treatment with NaCl. (a) Sodium hyaluronate (b) and 0.2% ambroxol (c). No obvious staining of fluorescein staining noted in rabbit corneas before operation (a1,b1,c1). Moderate degrees of patchy epithelial defects seen in the corneas two weeks after operation (a2,b2,c2). The rabbit corneas in NaCl group showed filamentary staining two weeks after treatment (a3) and moderate punctate staining 4 weeks after treatment (a4). The rabbit corneas in sodium hyaluronate group showed scattered punctate staining two weeks after treatment (b3) and minimal fluorescent staining four weeks after treatment (b4). The rabbit corneas in ambroxol group showed localized central punctate staining two weeks after treatment (c3) and hardly any staining four weeks after treatment (c4).
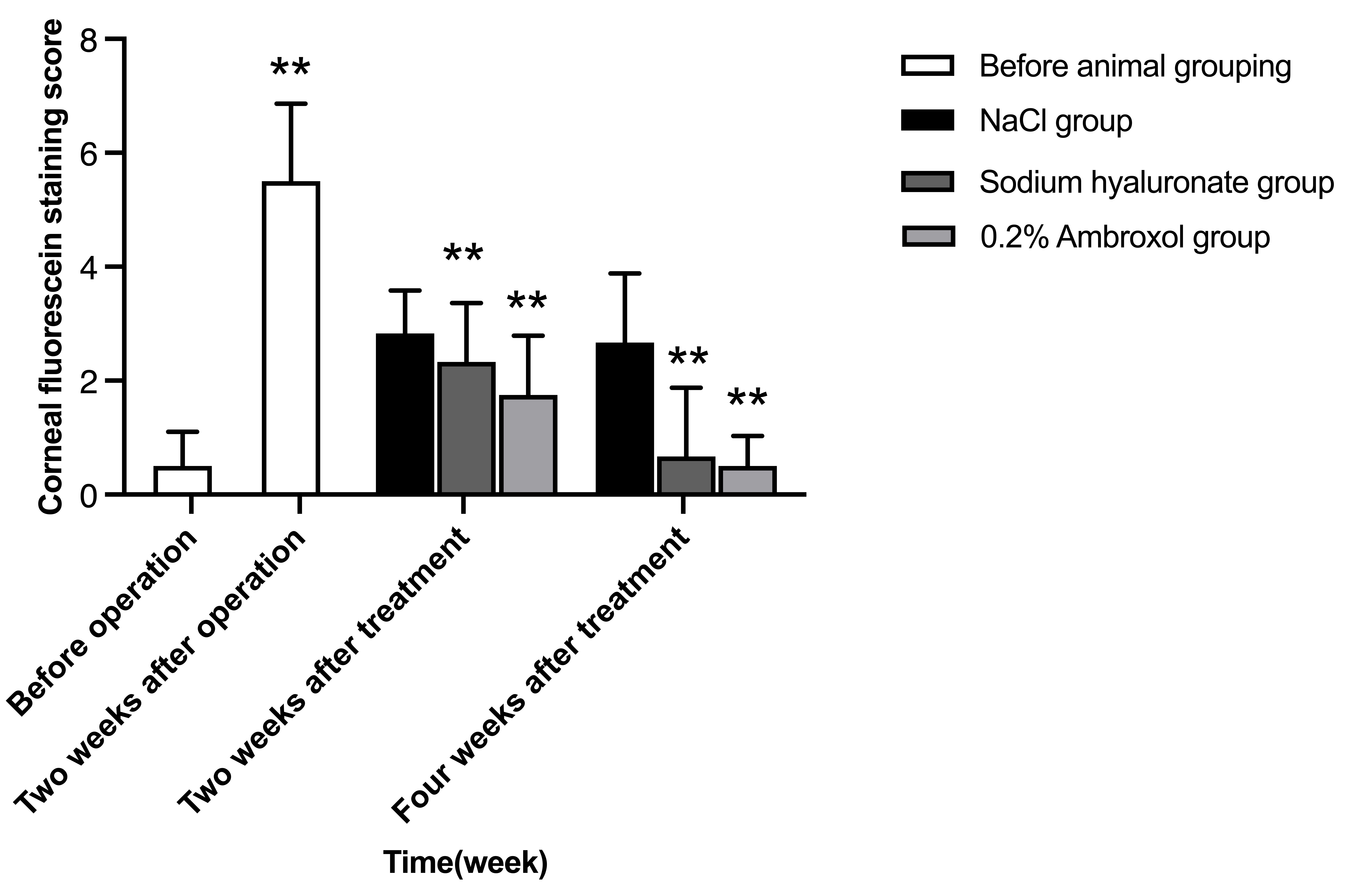 Fig. 4.
Fig. 4.Comparison of corneal fluorescein staining scores before
operation, two weeks after operation, two and four weeks after treatment among
groups. F (group) = 4.62, p
There was significant difference in tear secretion at different time in ambroxol
administration group (F = 6.34, p
Compared to two weeks after surgeries, two weeks after treatment, the corneal patchy fluorescent staining disappeared in ambroxol group, leaving only focal punctate staining. After two weeks of treatment, sodium hyaluronate group showed scattered punctate staining, whereas NaCl group showed filamentous staining. Four weeks after treatment, the fluorescent staining of corneas in ambroxol and sodium hyaluronate groups almost disappeared, whereas that in NaCl group still showed scattered punctate staining (Fig. 3).
Compared to two weeks after surgeries, the corneal fluorescein staining scores
in ambroxol and sodium hyaluronate groups were significantly reduced at two and
four weeks after the treatment (p
After four weeks of treatment with respective eye drops, the conjunctival
specimens were assessed by ELISA for level of TNF-
| Group | Eyes/group | Concentration of TNF- |
Concentration of IL-8 (ng/L) | Concentration of MUC5AC ( |
| Ambroxol | 8 | 1171.34 |
63.89 |
123.98 |
| NaCl | 8 | 1325.20 |
67.70 |
106.53 |
| Sodium hyaluronate | 8 | 1331.93 |
67.02 |
115.67 |
| F value | 7.08 | 2.50 | 13.10 | |
| p value | 0.11 | |||
| Note: Comparison of ambroxol with NaCl group and Sodium hyaluronate ,
*p | ||||
The results of oil red O staining of meibomian glands showed that after four weeks of treatment, compared with NaCl and sodium hyaluronate groups, there appeared to have more bright red oil content in the ambroxol group (Fig. 5).
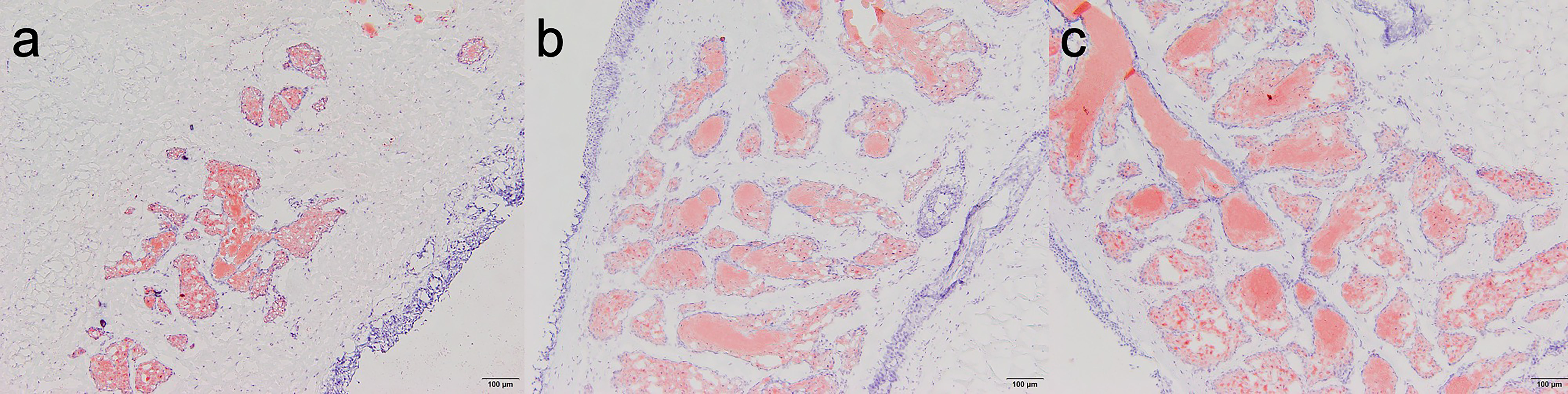 Fig. 5.
Fig. 5.Oil red O staining of rabbit eyelid meibomian glands among groups
four weeks after treatment (HE
According to the DEWS II 2017 report, dry eye is an ocular surface disease associated with multiple risk factors [14, 15]. The fundamental characteristic of DED is the disruption of the TF homeostasis, combined with various ocular symptoms. Instability of the TF, increased osmolarity and inflammation, damage to the ocular surface, decreased androgen level, and the neurosensory abnormalities all play important roles in the pathogenesis of DED [15]. Compared with rats and mice, the globe size, anatomical structure, physiological function of tissues and the histoarchitectural features of lacrimal glands in rabbits resemble more closely those in human [16]. In addition, rabbit eyes are more suitable to slit lamp microscope observation and photography. Hence, our group commonly use rabbits to create a mixed-mechanism model to study DED. In present study, two weeks after model creation, the Schirmer I test value had significantly decreased from the baseline by about 30%, and the corneal fluorescein staining score significantly increased. The reliability and reproducibility of this model is truly remarkable.
The TF is composed of water, lipid and mucus layers to protect the cornea from exogenous microorganisms, chemical substances and environmental pollutants, and to maintain a smooth refractive surface required for clear vision [17]. Hence, maintaining the homeostasis of the TF is the key in DED treatment.
Our earlier research suggested that augmentation of the AQP function in the conjunctiva has the potential to promote the secretion of tear and MUC5AC, the gel-forming mucin secreted by goblet cells [2, 11]. As an active agent on the ocular surface, MUC5AC can help evenly disperse the aqueous layer of the TF [18]. Aqueous and mucin deficiency both contribute to the etiology of DED [19, 20]. Ambroxol, an active metabolite of bromhexine, is a secretolytic and mucoactive drug primarily used to treat respiratory diseases associated with viscid mucus [4]. In addition to reducing the viscosity of mucus and stimulating the secretion of airway surfactants [5], ambroxol is also known to possess properties of anti-inflammation [6] and analgesia [7]. We have demonstrated recently that ambroxol can stimulate tear and mucin production in normal rabbits by upregulating the expression of AQP and MUC5AC, and hence may have the potential to be a DED drug [3]. The present study further tested the effect of ambroxol on the rabbit dry eye model and shed new light on mode of action by this drug.
In reference to data from previous studies, the present study chose to test 0.2% ambroxol in a mixed mechanism rabbit dry eye model, in comparison to NaCl and sodium hyaluronate groups as controls. Four weeks after treatment, 0.2% ambroxol indeed significantly promoted the secretion of tears on the ocular surface and the recovery of corneal epithelial injury. This study is significant since it is the first time efficacy for DED was demonstrated in vivo. Consistent with previous findings at tissue and cellular levels, ambroxol promoted the production of MUC5AC in the conjunctival tissue as well. Through histological examination of eyelids, the lipid component in the meibomian glands also appeared to have increased, as reflected by oil red O staining. Bromhexine, the pro-drug of ambroxol, has been shown to increase neutral lipid production in cultured conjunctival cells [21]. Its mechanism may be related to the up-regulation of CHML-mediated Rab7 expression which triggers the change of lysosomal activity and enhances the intracellular transport of many substances such as mucin and lipid droplets [21].
To further explore the mechanism of action, the present study assessed the
effect of ambroxol on inhibiting ocular surface tissue inflammation. Four weeks
after treatment, the levels of TNF-
In addition to reducing levels of key inflammatory cytokines such as
TNF-
Conjunctival epithelium regulates mucosal hydration mainly through the coupling process of sodium ion absorption mediated by cellular sodium channel and chloride ion secretion mediated by cystic fibrosis transmembrane conductance regulator (CFTR). The transconjunctival fluid transport powered by the osmotic pressure gradient is carried out by aquaporins. Evidence shows that ambroxol can up-regulate the expression of CFTR protein and enhance the transmembrane secretion of chloride ions on the surface of respiratory epithelial cells [26]. In addition to promoting the function of AQP molecule, we speculate that ambroxol in the conjunctiva could also stimulate the function of CFTR, thereby increasing the secretion of tears.
Ocular surface tissues, especially the conjunctiva, has sufficient compensatory capacity to generate tears required for tear turnover [27]. DED, especially that of non-autoimmune nature, can often recover spontaneously. In fact, severe recalcitrant DED often shows a significant loss of conjunctival goblet cells or end-stage keratosis [17], indicating that the conjunctival tissue is irreversibly damaged and the compensatory capacity seriously impaired. Therefore, early intervention to maintain the normal function of ocular surface tissues should be an important component in DED treatment. Diquafosol sodium and rebamipide, both promote the secretion of tears and mucin in dry eye patients. Diquafoxol sodium has a P2Y2 receptor agonist activity comparable to UTP [28]. Rebamipide is a quinolinone derivative with mucin secretagogue activity [29]. Ambroxol not only promotes the secretion of conjunctival tears, mucins, and even possibly lipids, but also has anti-inflammatory property, which are all desirable actions as a DED medicine.
Given that DED is a multifactorial disease which requires a multifaceted approach for management, drugs of multiple pharmaceutical properties obviously have more advantages over single action ones. Ambroxol distinguishes itself from other current single mechnisam drugs on the market or in the development pipeline, and is poised to be the next generation drug candidate for DED.
Ambroxol has the potentials to become the next generation multi action DED drug candidate. Further research exploration of the pharmaceutical properties of ambroxol is warranted in the future.
AQP5, aquaporin 5; ARVO, Association for Research in Vision and Ophthalmology;
CFTR, cystic fibrosis transmembrane conductance regulator; DED, dry eye disease;
DEWS II, Dry Eye Workshop II; ELISA, enzyme linked immunosorbent assay; ENaC,
epithelial Na
LY, MW and SZ were responsible for experimental design, interpretation of study results and drafting of the manuscript for publication. LY, ZW (Zixuan Wang) and ZW (Zhenhan Wang) were responsible for study execution and statistical analyses.
The protocol was approved by Shenzhen Eye Hospital Institutional Animal Care and Use Committees. The study was conducted in compliance with the Tenets of the Declaration of Helsinki and ARVO statement for the use of animals in ophthalmic and visual research.
Not applicable.
Free Exploration Project of Shenzhen Science and Innovation Commission (JCYJ20170306140638792); International Cooperation Project of Shenzhen Science and Innovation Commission (GJHZ20180929145202083); Medical Prevention Combined Ophthalmology Project. Supported by Shenzhen Fund for Guangdong Provincial High-level Clinical Key Specialties (No. SZGSP014).
Mingwu Wang holds international patents in the US, Canada, Australia, Korea, Japan and India, for topical application of ambroxol for treatment of ocular surface diseases. The other authors have no commercial or proprietary interest in any concept or products described in this article.
