†These authors contributed equally.
Academic Editor: Graham Pawelec
Objective: Postoperative complications of surgical revascularization in
moyamoya disease (MMD) are difficult to predict because of poor knowledge of the
underlying pathophysiological process. Since the aim of surgery is to improve
brain dynamics by increasing regional blood flow, we hypothesize that
postoperative complications are closely related to aberrant electrophysiological
changes. Thus, we evaluated the clinical significance of intraoperative
electrocorticography (iECoG) in bypass surgery for adult MMD. Methods:
Ninety-one adult patients operated on by the same neurosurgeon in our institute
were involved (26 in the iECoG group, 65 in the traditional group). Two 1
Moyamoya disease (MMD) is characterized by progressive stenosis or occlusion of the distal internal carotid artery or proximal middle cerebral artery, and it leads to the formation of abnormal collateral vessels at the base of the brain. Surgical revascularization, including direct, indirect, and combined bypass, has been confirmed to benefit MMD patients with both ischemic and hemorrhagic stroke episodes [1]. Nevertheless, postoperative complications such as cerebral ischemia, epilepsy, and hyperperfusion syndrome, have been increasingly reported [2, 3]. These complications may prolong hospitalization and be irreversible but can hardly be predicted by our present knowledge and experience [4].
Electroencephalography (EEG) has a high temporal resolution and is therefore suited to evaluating variations in neural activity after an event [5]. Previous studies have shown that alpha power reduction in continuous quantitative EEG predicts delayed cerebral ischemia, reflects successful therapy and predicts good functional outcome after subarachnoid hemorrhage [6, 7]. Based on these findings, several studies indicate that EEG can promptly detect cerebral responses to successful reperfusion therapy [8]. Intraoperative electrocorticography (iECoG) is a newly developed EEG monitoring method with a higher sensitivity and accuracy and has been widely used in neurosurgery to help distinguish high-frequency oscillations continuously and noninvasively [9]. Its indices, such as relative delta power, delta/alpha power ratio (DAR), and delta+theta/alpha+beta power ratio (DTABR), are associated with ischemic lesions [10]. Finnigan et al. [11, 12] believed that the delta/alpha power ratio (DAR) demonstrated maximal accuracy for discriminating between acute ischemic stroke patients and controls.
Currently, successful bypass surgery is still evaluated by the patency of anastomosis rather than the rate of postoperative complications. It’s vital for us to realize that effectiveness of bypass is based not only on hemodynamic remodeling, but on functional re-plasticity as well. Clinical experience indicates that patent anastomosis and sound clinical outcomes cannot always be achieved simultaneously. Poor knowledge of neural interactions before and after surgical revascularization may be the main reason. Thus, the present study evaluated the application of iECoG in adult MMD and tried to explore the effectiveness of bypass surgery from the perspective of electrophysiology.
All patients with MMD who were admitted to our hospital between May and August 2018 were consecutively enrolled in this study. Our study used historical controls that included 65 hemispheres in 65 patients who received the same operation without iECoG monitoring between January and May 2018. Inclusion criteria were (1) a Chinese population aged 18–65 years; (2) no evidence of infarction history, but small patches of hyperintense signal that were neither larger than the arbitrary cutoff of 8 mm in maximum dimension on T2-weighted MR images nor cystic in the cerebral subcortical white matter; (3) diagnosis through digital subtraction angiography; (4) no surgery before recruitment; (5) physically capable of cognitive evaluation; (6) meet the surgical indications of guidelines for MMD [4] and (7) absence of significant psychiatric disorders or neurological diseases that could compromise cognition. Patients with severe systemic or other cerebrovascular diseases and those taking drugs were excluded.
All patients underwent a combined revascularization procedure in which the superficial temporal artery was anastomosed to the M4 portion (cortical segment) of the middle cerebral artery near the Sylvian fissure and encephaloduromyosynangiosis (EDMS) surgery. The patency of the bypass artery was confirmed using indocyanine green (ICG) angiography. We used intraoperative cortical electrodes for electrophysiological monitoring, and the power spectral density (PSD) of each frequency band was calculated in real time. When selecting the recipient artery out of the two or more candidate arteries, we selected the artery closer to the cortex with lower PSD in the high-frequency bands according to the iECoG recordings and real-time PSD calculation. During the direct revascularization procedure, iECoG data were recorded continuously until the dura incision. The study protocol was approved by the ethics committee of Huashan Hospital, China, and all subjects provided informed consent (Fig. 1).
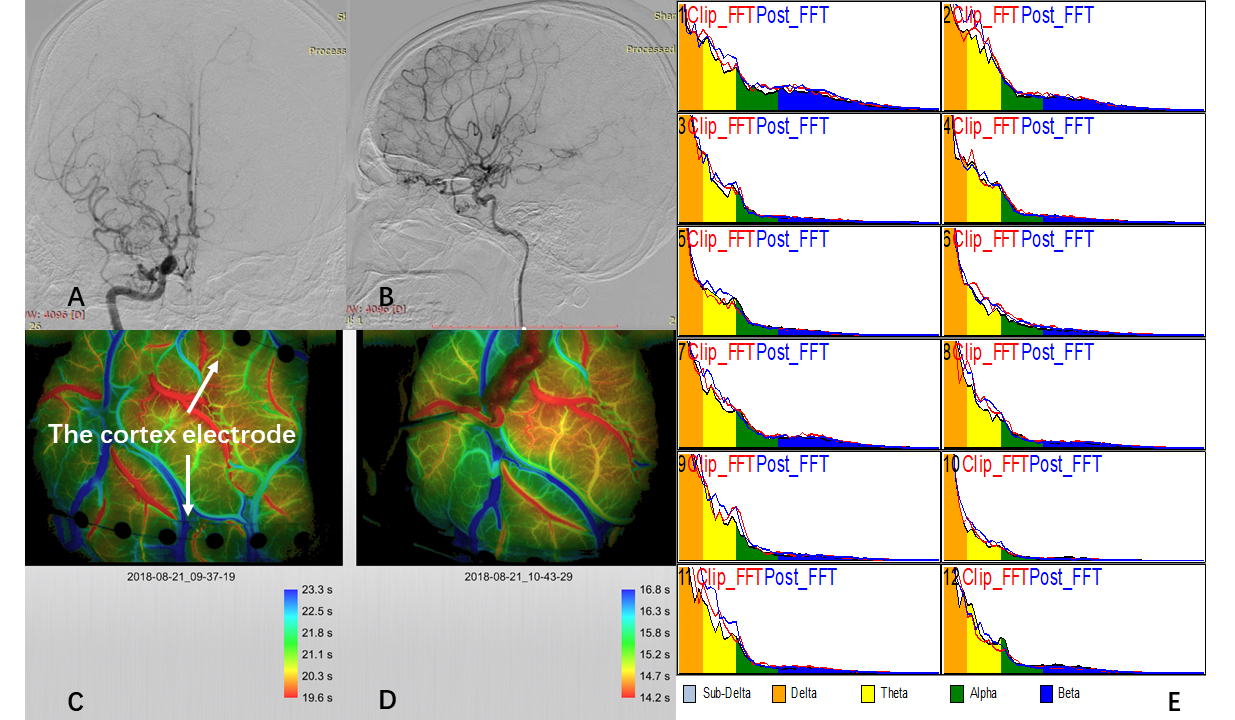 Fig. 1.
Fig. 1.ICG and iECoG monitoring for modified revascularization. The
diagnosis of moyamoya disease (MMD) was confirmed by digital subtraction
angiography (DSA) (A,B). Two 1
Baseline examinations involved taking a medical history and carrying out a physical examination. Neurological deficits and cognitive performance were quantified using the National Institutes of Health Stroke Scale (NIHSS) and Mini-Mental State Examination (MMSE) at arrival at the neurosurgery department and at 1 month after anastomosis. Perioperative complications, including cerebral hemorrhage, cerebral infarction, epilepsy and transient neurological events (TNEs), were confirmed by imaging examination and nervous system physical examination.
All patients received inhalation anesthesia administered by anesthesiologists,
with end-tidal sevoflurane maintained at 2% during the iECoG recording. The
iECoG data were recorded with a BrainAmp MR PLUS (Brainproduct, Munich, Germany)
using a preamplifier bandwidth of 0.5–250 Hz and a sampling rate of 500 Hz. Two
1
We selected the three electrodes from the two 1
The PSD is deterministic, and for certain types of random signals, it is independent of time. This is useful because the Fourier transform of a random time signal is itself random and therefore of little use in calculating transfer relationships (i.e., finding the output of a filter when the input is random). The PSD of a random time signal x(t) can be expressed in one of two ways that are equivalent to each other.
(1) The PSD is the average of the Fourier transform magnitude squared over a large time interval.
(2) The PSD is the Fourier transform of the autocorrelation function.
The power can be calculated from a random signal over a given band of frequencies as follows:
(1) Total Power in x(t):
(2) Power in x(t) in the range f1–f2:
Data in this study were derived from the mean values of the three electrodes in the bypass area and the three in the nonbypass area. For each electrode over the range of 1–30 Hz, the power of the delta (1–3 Hz), theta (4–7 Hz), alpha (8–13 Hz) and beta1 (14–20 Hz) frequency bands over all channels were used to calculate the global DTABR.
Once the electrode grids were placed on the cortex, data were recorded and labeled as “Pre”. After the anastomosis was finished and temporary blocking clips of recipient artery were removed, data were labeled as “Post”. A period of interference-free waves was picked out for each period and pre- and post-bypass PSD were analyzed separately. Artifacts caused by electronic devices and motion were removed from the raw ECoG data by two neurosurgeons. We first compared the PSD of patients between pre- and post-bypass phases in the bypass area. To compare the spectral power of each band, the ratio of the spectral power of each band to that of the whole was calculated as follows: X/DTAB = (spectral power of each band)/(sum of spectral power of 4 bands: delta, theta, alpha, beta), where X is the spectral power of the delta, theta, alpha or beta band.
For both the bypass and nonbypass areas, we then compared the PSD for each frequency band and the DTABR between the pre- and post-bypass phases. To detect the changes in each parameter before and after surgery, the ratios between postoperative and preoperative values were used.
The incidences of perioperative complications (cerebral infarction, epilepsy,
and cerebral hyperperfusion syndrome) were compared between the two groups (MMD
patients who received bypass surgery with and without iECoG monitoring). Data
were compared between the two groups using the
A total of 26 patients with MMD were examined. The mean age of these patients
was 45.38
| Characteristics | iECoG group | Control group | p value |
| Number | 26 | 65 | |
| Age | 45.38 |
45.18 |
0.94 |
| Sex (Male) | 12 | 30 | 0.569 |
| Perioperative complications | |||
| Hemorrhage | 0 | 1 | 0.714 |
| CI | 1 | 2 | 0.64 |
| EP | 3 | 7 | 0.916 |
| TNEs | 1 | 13 | 0.046* |
| Total | 5 (19.2%) | 23 (35.3%) | 0.103 |
| Baseline NIHSS | 1.615 |
1.769 |
0.731 |
| △NIHSS | –0.077 |
–0.3078 |
0.478 |
| Baseline MMSE | 24.04 |
23.58 |
0.349 |
| △MMSE | 0.539 |
0.785 |
0.5523 |
| CI, cerebral infarction; EP, epilepsy; TNEs, transient neurological events; NIHSS, National Institutes of Health Stroke Scale; MMSE, Mini-Mental State Examination. | |||
The mean values of PSD in the pre- vs. post-bypass phases in the bypass area
were as follows: D/DTAB, 0.54
Fig. 2 shows the correlations between the post-/pre-bypass PSD parameter ratios
for each frequency band and the change in NIHSS score. The post-/pre-bypass DTABR
was significantly correlated with the change in the NIHSS score (p =
0.002, r
 Fig. 2.
Fig. 2.Correlations between the post-/pre-bypass PSD and DTABR ratios for the bypass area and NIHSS score change. PSD, power spectral density, DTABR: (delta+theta)/(alpha+beta) ratio; NIHSS, National Institutes of Health Stroke Scale.
Fig. 3 shows the correlations between the post-/pre-bypass PSD parameter ratios
for each frequency band and the change in cognitive performance, as assessed by
MMSE scores. The post-/pre-bypass DTABR decreased as the MMSE score increased
(p = 0.007, r
 Fig. 3.
Fig. 3.Correlations between the post-/pre-bypass PSD and DTABR ratios for the bypass area and MMSE score change. PSD, power spectral density; DTABR, (delta+theta)/(alpha+beta) ratio; MMSE, Mini-Mental State Examination.
We also analyzed correlations between the post-/pre-bypass PSD parameter ratios for each frequency band in the nonbypass area and changes in neurological and cognitive performance. Neither the NIHSS nor the MMSE score was correlated with indices of intraoperative cortical electrophysiology (Figs. 4,5).
 Fig. 4.
Fig. 4.Correlations between the post-/pre-bypass PSD and DTABR ratios for the nonbypass area and NIHSS score change. PSD, power spectral density; DTABR, (delta+theta)/(alpha+beta) ratio; NIHSS, National Institutes of Health Stroke Scale.
 Fig. 5.
Fig. 5.Correlations between the post-/pre-bypass PSD and DTABR ratios for the nonbypass area and MMSE score change. PSD, power spectral density; DTABR, (delta+theta)/(alpha+beta) ratio; MMSE, Mini-Mental State Examination.
We further compared the post-/pre-PSDs and DTABR ratio among patients with and
without postoperative complications. In the bypass area, the DTABR ratio (1.67
 Fig. 6.
Fig. 6.Differences in the DTABR ratio and PSD of each frequency band in the bypass area between the complication and noncomplication groups. Significant differences were found in the DTABR ratio and PSD of the theta band. PSD, power spectral density; DTABR, (delta+theta)/(alpha+beta) ratio.
 Fig. 7.
Fig. 7.Differences in the DTABR ratio and PSD of each frequency band in the nonbypass area between the complication and noncomplication groups.
Our results quantitatively confirm previous findings in which the PSD of the
In line with previous studies [14, 15, 16, 17, 18], our results demonstrated that the PSD of high-frequency waves increased, while the PSDs of the low-frequency bands were attenuated after bypass surgery. This provides new evidence for the physiological significance of EEG recordings with respect to cerebral perfusion.
The process of revascularization is rapid, occurring within a few minutes to a few days [19, 20, 21]. Zhang et al. [4] have confirmed the clinical significance of hemodynamic parameters change in bypass surgery to predict perioperative complications, which has a high spatial resolution. While, neural electrophysiological monitoring can reflect changes in cerebral blood flow and metabolism within seconds, as these factors are directly reflected in neuronal rhythms with high temporal resolution [22]. The high temporal resolution of EEG permits sensitive assessment of instantaneous brain functioning [23, 24]. EEG spectrum frequencies associated with acute cerebral ischemia have been discussed [11, 17], but there are few reports on QEEG characteristics related to bypass surgery.
Interestingly, in our study, not all cases showed an increase in high-frequency PSD and a decrease in low-frequency PSD. Cerebral hemodynamic changes after anastomosis are complicated, and hemodynamic stability is difficult to maintain during combined revascularization.
The functional role of the overall change in high-frequency PSD remains unclear. In a recent review, the beta band was associated with response inhibition. Beta oscillatory responses were also found to be positively related to somatosensory and motor functions. In another study, beta band activity reflected arousal of the visual system during increased visual attention. For the gamma band, gamma rhythms are believed to represent the binding together of different neuronal populations into a network to carry out a certain cognitive or motor function. The earliest model of gamma oscillations was based on reciprocal connections between pools of excitatory pyramidal and inhibitory neurons. In another model of these neurons, axon conduction and synaptic delays lead to a phase shift between pyramidal and interneuron spikes, and these delays determine the frequency of the gamma rhythm [13].
The change in DTABR was significantly correlated with NIHSS score improvement. This supports the use of iECoG parameters (such as DTABR) in prognostication regarding MMD patients undergoing revascularization surgery, which is in line with previous findings. In 26 patients with subacute ischemic stroke in the middle cerebral artery territory, Z-values of absolute delta power (derived using a normative population database) displayed a higher prognostic value compared to the Canadian Neurological Scale regarding 3-month stroke outcomes measured by the modified Rankin Scale [25]. Finnigan et al. [14] obtained the acute delta change index (aDCI) from two recordings and compared it to serial diffusion-weighted magnetic resonance imaging (DWI) and perfusion-weighted magnetic resonance imaging (PWI) data from 11 patients within 16 h after the onset of confirmed stroke symptoms. The correlation between aDCI and the 30-day NIHSS score was higher than that between aDCI and initial DWI lesion volumes [14]. Furthermore, in 13 patients with subacute ischemic stroke, DAR and relative alpha power were significantly correlated with the 30-day NIHSS score. Finnigan et al. [14] also found the DAR to be the most effective QEEG measure for prognostication regarding the evolution of cerebral ischemia. In comparison to the DAR, the DTABR evaluates theta and beta activity as well, both of which may be altered by strokes [26, 27, 28].
In our study, there was a significant difference in the incidence of postoperative transient neurological dysfunction between the precision bypass group and the traditional group, while no significant difference was found in the incidence of postoperative complications such as cerebral infarction and epilepsy. The phenomenon of hyperperfusion syndrome is one of the causes of transient neurological dysfunction after direct bypass in MMD. In the precision bypass group, a relatively low perfusion area was selected for STA-MCA bypass surgery through iECoG monitoring, which may avoid local blood flow overperfusion and thus reduce the occurrence of postoperative TNEs. In the course of iECoG monitoring, we found that some patients had sporadic epileptiform discharge, which may be related to the occurrence of postoperative epilepsy. Our finding was expected because accumulating data suggest that high-frequency oscillations are biomarkers for the epileptogenic zone in nontumoral epilepsies [29]. However, under the premise that the clinical significance of this phenomenon was not clear, we did not make special adjustments to postoperative antiepileptic therapy. We believe that if the occurrence of postoperative epilepsy can be predicted by iECoG monitoring and if drug intervention can be carried out in advance, then the incidence of postoperative epilepsy can also be effectively controlled.
The results indicated that the post-/pre-bypass DTABR ratio was significantly higher among the patients with postoperative complications than among those without complications. The increase in postoperative DTABR indicates an increase in postoperative cortical ischemia. Although combined bypass surgery clearly has an immediate positive effect on cerebral hemodynamics, postoperative hemodynamic instability still needs more study. Using QEEG, we can not only predict the occurrence of postoperative complications quantitatively but also provide a reliable and effective tool for future research.
As a pioneer study to explore the correlation between iECoG and bypass surgery, our study had several limitations. The first was the small sample size of 26 patients, which led to insufficient statistical power. We believe that increasing the sample size in a future study will increase the statistical significance of the existing indicators and allow more indicators to be discovered. Another possible limitation of our study was the relative stationarity of the iECoG recordings. Intraoperative influences, including the use of a bipolar electric coagulation knife, can be removed artificially during data processing but cannot be completely avoided. The intrinsic PSD characteristics of different brain regions may affect the intraoperative judgment of cortical hypoperfusion regions, which may partly explain why the overall perioperative complication rate in the precision bypass group was not significantly lower than that in the conventional group. Due to the intrinsic properties of the evaluated iECoG parameters, there are some differences in cortical electrical activities or frequency power between awake patients and anesthetized MMD patients. What’s more, there is few literatures specifically investigating the relationship between perfusion status and frequency bands in the setting of anesthesia, which need further researches. Finally, we were unable to compare the ischemic stroke-related EEG effects between affected and unaffected hemispheres and to assess their correlations with functional outcomes.
Our study confirmed that cortical electrophysiological monitoring could sensitively reflect changes in cortical blood flow during bypass surgery. QEEG parameters have good predictive value for the improvement of postoperative neurological function, neuropsychological performance and the occurrence of complications in patients with MMD. Cortical electrophysiological monitoring could be used as a potential reference for vascular selection in patients undergoing bypass surgery.
MMD, moyamoya disease; iECoG, intraoperative electrocorticography; PSD, power spectral density; DTABR, (delta+theta)/(alpha+beta) ratio.
XZ and JS performed all data acquisition and interpretation and drafted the manuscript. JY assisted with data interpretation and revised the manuscript. YJL, SY, HY and CG assisted with data collection. WN, YL, RF and YG guided article revision. All authors contributed to the article and approved the submitted version.
All procedures in this study were conducted in accordance with the Institutional Review Board in Huashan Hospital, Fudan University, China (approval No. 2014-278). Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Thanks to all the peer reviewers for their opinions and suggestions.
This study was supported by the National Natural Science Foundation of China (Nos. 81771237 and 81801155), the Shanghai Municipal Science and Technology Commission Major Project (Nos. 2018SHZDZX03 and 19DZ1930304), ZJLab, and the Shanghai Municipal Commission of Health and Family Planning (Nos. 2017BR022 and 2019SY076).
The authors declare no conflict of interest.
