Academic Editor: Elena Levantini
Background: Recently, the incidence of hematological malignancy, such as various leukemias, multiple myeloma and lymphoma, has revealed an increasing tendency, exhibiting a major impact on human health. Most of the available anti-cancer drugs, however, possess high non-targeted accumulation, dosage-associated toxicity, fast elimination, and lack specificity towards tumors, which restrict their utilization in clinical therapy. This extends also to cancer diagnosis where there is a lack of predictive biomarkers. Object: Noble metal nanomaterials (NM NMs) have the potential to overcome these shortcomings due to several characteristics including ease of synthesis, ultra-small size, easy surface modification and specific physicochemical properties. At present, gold-, silver- and platinum-based nanomaterials have been employed in the tracing and treatment of hematopoietic tumors through direct individual endocytosis or in innovative drug delivery systems (DDS) by conjugation with other targeting biomolecules. Purpose: In this mini review, we focus on the use of localized surface plasmon resonance (LSPR)-/surface-enhanced Raman scattering (SERS)- and fluorescence-based diagnosis of NM NMs in the hematological malignancies. Furthermore, the treatment of hematological malignancies utilized the NM NMs or NM NMs-based therapy technology in the chemotherapy, targeted therapy, and photothermal therapy are depicted in depth. The construction of effective and promising NM NMs or NM NMs- dependent theranostic methodology has the potential to provide interdisciplinary knowledge in the development of clinical tracing, diagnosis and treatment of refractory hematological diseases.
Cancer as the one of three challenges of modern medicine is reported to be the leading cause of human mortality and the main hindrance to prolonging life expectancy worldwide in the 21st century [1]. Hematologic malignancies represent malignant tumors of bone marrow, hematopoietic and lymphoid tissues, accounting for 7.2% of total deaths and 6.5% of new cancers, based on global cancer statistics in 2018 [2]. Generally, hematological malignancies are divided into three types, namely leukemias, lymphomas and myelomas, and each of these contain multiple subtypes [3, 4, 5]. Leukemias include acute lymphoblastic leukemia (ALL), acute myelogenous leukemia (AML), chronic lymphocytic leukemia (CLL), chronic myelogenous leukemia (CML), acute monocytic leukemia (AMoL) and other less common subtypes [6]. Lymphomas contain non-Hodgkin’s lymphoma and Hodgkin’s lymphoma [7], while myelomas are divided into myelodysplastic syndrome (MDS) and myeloproliferative neoplasms (MPN) [8]. Among these, leukemia is associated with high mortality and morbidity rates in comparison to other subtypes, and three quarters of the cases occur in childhood [6, 9, 10].
Over the past several years, hemopathology has gained a great development, especially the emergence of numerous new technologies for the diagnosis of hematological malignancies [11]. The World Health Organization (WHO) has reported a complex classification standard for hematological malignancies, which makes the diagnosis more difficult [3, 12]. The increased use of morphology, immunology, genetics and molecular biology (MIGM) has become important diagnostic methodologies for various hematological malignancies [13]. On the other hand, the treatment of hematological malignancies has also been gradually improved, resulting in significant improvement in complete remission (CR) rates, disease-free survival (DFS) rates and overall survival (OS) rates for patients [14]. Although induction differentiation therapy, autologous stem-cell transplantation (SCT), biological immunotherapy, targeted and gene therapy have developed rapidly recently, chemotherapy and radiotherapy are still predominantly used for the treatment of hematological diseases (Fig. 1) [4, 7, 15, 16]. However, the traditional combined treatment with chemotherapy and radiotherapy leads to discomfort and irreversible side effects such as hair loss, fatigue, nausea, even infection and organ damage [17, 18, 19]. Patients with hematologic malignancy receiving chemotherapeutic drugs have the complication of febrile neutropenia, which dramatically increases the infection rate and mortality [20]. Besides, the pharmacokinetics of chemotherapeutic drugs are suboptimal, leading to inevitable relapse and reduced limitation to their clinical application. Bone marrow transplantation as one form of stem cell transplantation (SCT) represents another choice to cure different hematological malignancies, which has the capacity to achieve better survival rates [21]. Rare match type, few relevant patients and likely complications including graft versus host reaction (GVHD), recurrence, infection, and end organ dysfunction, create prominent resistance to the therapeutic process [22, 23]. Certainly, some patients are not eligible for SCT or frequently relapse after SCT, therefore, novel treatments still need to be intensively explored.
 Fig. 1.
Fig. 1.The schematic principles for treatment of hematological malignancies.
Monoclonal antibodies alone or combined with chemotherapy, referred to as immunotherapy, have introduced an innovative therapeutic regimen for hematologic malignancies as depicted in Fig. 1 [24, 25, 26, 27]. A representative monoclonal antibody is rituximab which has been shown to improve the clinical outcome and efficiently decrease the mortality for the patients with B-cell malignancies [28]. Moreover, some monoclonal antibodies such as bispecific T cell engagers (BiTEs), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor, and programmed cell death protein 1 (PD-1) inhibitor, regulate the activation of T cell via the histocompatibility-antigenic peptide complex that expresses a chimeric antigen receptor (CAR), resulting in the eradication of tumor and strengthening of immune response [4, 29, 30, 31, 32]. Despite the fact that immunotherapy provides considerable hope for patients, these T-cell-engaging therapies are also associated with certain toxicity [33, 34]. With the development of molecular biologicals, genomic and nano technology, targeted therapy construction of suitable drug delivery systems has become more efficient, safe and represent pharmacokinetic treatment alternatives. Integration of targeted drugs into conventional chemotherapy, radiotherapy and SCT, has become an innovative breakthrough in the traditional treatment of patients with various refractory tumor [35, 36]. These drugs have a targeted effect on the site of the neoplasm and impede the biological transduction pathways and/or certain oncoproteins to induce the death of carcinoma cells by immune system stimulation or apoptotic effects. Recently, considerable progress has been made on the discovery of targeted therapeutic methods for hematologic malignancies. For example, employing CD20 monoclonal antibodies, fms-related tyrosine kinase 3 (FLT3) inhibitor, and tyrosine kinase inhibitor to target therapy for B-cell lymphoma and CD20 positive leukemia [37], FLT3 positive and high-risk AML [38] and CML [39], respectively. Nevertheless, only few reports showed complete cure or acquired long-term remission due to short biological half-life in circulation and damage to healthy tissues [40]. Chemotherapeutic drugs encapsulated by nano-carriers like liposomes and polymeric micelles, have an ability to retain active substances during transportation towards malignant cells and reduce the exposure of drugs to normal tissues, which has been successfully implemented in some clinical reports [41, 42, 43].
In the past decades, metal nanomaterials (M NMs) have received extensive
attention in the fields of electronics, catalysis, optics, and biology [44, 45, 46].
When the size of bulk metal compounds is decreased to the nano range, dramatic
physical and chemical properties change due to the quantum size effect, surface
effect, and macro-quantum tunnel effect [47]. Metal nanoparticles (M NPs) are
generally defined as particle-aggregates with sizes between 2 and 100 nanometers
[48, 49]. As the free electrons are confined related to Fermi wavelength (
Early and accurate diagnosis of malignancy is one of the most critical points for patients to alleviate mental pain and economic burden [44]. Current cell-based diagnostic tools such as immunocytochemistry, anatomical imaging, pathological examination require sufficient malignant cells, which are usually evident in advanced stages of the disease. X-ray imaging, computerized tomography (CT), positron emission tomography (PET), magnetic resonance imaging (MRI) as the classical anatomical imaging techniques which are associated with intense ionizing radiation or electromagnetic radiation can be harmful to humans [61]. Finding a less harmful alternative tissue imaging methodology in monitoring of cancer is an important issue. Body fluids (blood, urine, saliva) contain potential biomarkers associated with the evolution of cancer cells, such as proteins/peptides, microRNAs (miRNAs), exosomes, circulating tumor DNA (ctDNA), circulating tumor cells (CTCs) represent an approach to such imaging [62, 63, 64, 65, 66, 67]. Establishing a rapid and non-invasive approach instead of standard tissue biopsy to test the prognosis agents mentioned above, even in a low concentration, could reduce the risk of metastasis and detect cancer evolution at early stages. In general, NM NMs with unique physicochemical properties and low toxicity are normally employed as sensitive probes or safe contrast agents to achieve the analyte detection and cellular imaging of tumors on account of their specific localized surface plasmon resonance (LSPR), surface-enhanced Raman scattering (SERS), and size-dependent fluorescence.
Since the light is incident to the surface of noble metallic
NPs, the conduction electrons exhibit specific collective
oscillations leading to the emergence of a strong optical absorption and/or
scattering peak as shown in Fig. 2A [68]. This unique phenomenon is termed LSPR
entirely affected by the NPs’ size, shape, dielectric environment and other
properties NM NPs, therefore, are frequently used as contrast agents for
microscopic imaging or as targeted probes for detection and recognition of tumor
cells dependent on the shifts of LSPR spectral peak when the diagnostic objects
approach the surface of NPs [69, 70]. For example, screening blood samples for
leukemia was proposed using nanohole-arrays on plastic (NAP) as a plasmonic
sensor and human immunoglobulin kappa and lambda light chains in blood serum as a
screening compound [71]. NAP was fabricated by the UV nanoimprinting on thin gold
films to form the nanohole arrays. This typical SPR-based nanosensing platform
has a capacity to accurately examine the overexpression of light chain antibodies
in cancerous blood samples. Cytokines as one kind of
immunomodulating protein biomarker, are secreted from immune cells and control
cell growth, cell differentiation and immune response [72]. The classical
enzyme-linked immunosorbent assay (ELISA) and fluorescent-based detection of
cytokines have several disadvantages like time-consuming, complexity, and large
sample consumption. A LSPR optofluidic platform device has been integrated with a
polydimethylsiloxane (PDMS) supporting layer, a microfluidic layer which traps
and incubates cells, and an LSPR sensing layer consisting of Au NPs connected
with tumor necrosis factor (TNF)-
 Fig. 2.
Fig. 2.The schematic principle for localized surface plasmon resonance (LSPR), and surface-enhanced Raman scattering (SERS). (A) Since the light is incident to the surface of noble metallic NPs, the conduction electrons exhibit specific collective oscillations leading to the emergence of a strong optical absorption and/or scattering peak. (B) Raman scattering signal is remarkably enhanced when the molecules are located on the surface of metallic naoparticles. Reprinted major modification with permission from [70], Copyright 2011, American Chemical Society.
If molecules are located on the surface of metallic NPs, the Raman scattering signal is remarkably enhanced, which is defined as SERS (Fig. 2B) [70, 74, 75, 76, 77, 78]. SERS technology offers a new strategy for both biomolecule detection and intracellular imaging [61]. In addition to high sensitivity, SERS can precisely recognize molecular structure as well as avoid interference from cellular autofluorescence [79]. Lentini et al. [80] constructed Ag NPs assembled phage clone (Ag NPs–EIII1) network as a SERS probe to identify the human histiocytic lymphoma U937 cells in vitro. Once Ag NPs-EIII1 targeted to the U937 cells, a new Raman scattering peak and enhancement intensity appeared, due to the existence of oligosaccharide complexes by means of molecules involved in probe-target interaction. Boca’s group initially prepared hollow gold-silver nanoparticles (HNS) and then mixed them with Nile Blue (NB) Raman reporter, modified by PEG to conjugate with antiCD19 monoclonal antibodies [81]. Consequently, SERS active molecules, HNS-NB-PEG-antiCD19, were produced and used for evaluating the uptake and intracellular distribution inside both CD19-positive SKW6.4 cells (Epstein-Barr virus-transformed B lymphocytes, B-cell lymphoma) and CD19-negative OCI-AML3 cell line (AML). The results revealed that HNS-NB-PEG-antiCD19 could selectively targeted and imaged the CD19-positive lymphoma cells, showing the potential for a luciferous assay in the early and precise diagnosis of lymphoblastic cancers.
In summary, LSPR-/SERS-based diagnostic strategies provide sensitive identification of neoplastic indicator and noninvasive imaging of hematologic cells, designed to understand and improve the treatment of hematologic system diseases in advance.
Among various types of diagnostic methods, fluorescence-based techniques exhibit
a highly sensitive, specific, and time-saving approach for the detection and
imaging of various cancers. To achieve the fluorescence-based diagnosis involves
combining the effective fluorescent agents such as organic dyes [82], fluorescent
proteins [83], semiconductor quantum dots (QDots) [84], carbon nanotubes (CNTs)
[85]. Novel NM NCs with discrete electronic state possess molecular-like
behaviors and exhibit size-dependent fluorescence from visible to the near
infrared (NIR) region [50]. In contrast to classical fluorophores, ultra-fine Au,
Ag and Pt nanoclusters (NCs) have stable photobleaching, strong luminescence, and
excellent biocompatibility, which has led to great progress in the application of
biomolecular detection and cellular labeling for hematological malignancies.
Tan’s group firstly reported the facile production of multidentate
thioether-terminated poly (methacrylic acid) (PTMP-PMAA) stabilized NIR
fluorescent Au NCs with 660 nm emission wavelength (Fig. 3A) [86]. These Au NCs
were able to bio-label both adherent HeLa cells and suspended Jurkat cells. More
interesting was the observation that hematopoietic cancer K562 cells had a
distinct tendency for internalizing more Au NCs than normal cord blood
mononuclear cells (CBMC), exhibiting a great potential application in diagnostic
detection of hematologic malignancies due to their selective affinity to enter
relative mature cells such as granulocytes and lymphocytes (Fig. 3B) [86].
Fluorescent glycine dimers capped Ag NPs with the sizes ranging
from 9 to 32 nm have been produced (Fig. 3C) [87]. This blue fluorescent
NPs-ligand system has a high quantum yield of (5.2
 Fig. 3.
Fig. 3.The fluorescence-based diagnosis of hematological malignancies using metal nanomaterials. (A) 3D excitation and emission spectrum of multidentate thioether-terminated poly (methacrylic acid) (PTMP-PMAA) stabilized near-infrared (NIR) fluorescent gold nanoclusters (Au NCs) and (B) confocal microscopic images of human umbilical cord blood mononuclear cells (CBMC) and hematopoietic cancer K562 cells cultured with Au NCs for 24 h. Cell nuclei were stained by Hochest 33258. Reprinted major modification with permission from [86], Copyright 2011, American Chemical Society. (C) Photoluminescence emission spectra of Ag NPs with different sizes excited by 366 nm UV light and (D) confocal microscopic graphs under cross-section (z-stacks) and top view of rat basophilic leukemia cells imaged by synthesized Ag NPs. Reprinted major modification with permission from [87], Copyright 2016, Springer. (E) 3D excitation and emission spectrum of polyethylenimine (PEI)-encased platinum nanoclusters (Pt NCs@PEI) and (F) confocal microscopic images of hematopoietic cancer K562 and BV173 cells as well as peripheral blood mononucleated cells (PBMCs) cultured with Pt NCs@PEI. Cell nuclei were stained by 6-diamidino-2-phenylindole (DAPI). Reprinted major modification with permission from [94], Copyright 2018, Elsevier.
With the rapid development of nanotechnology, NM NMs have the potential to play a crucial role in therapeutic applications for various cancers such as lung carcinoma, prostatic carcinoma, hepatocellular carcinoma and for other tumors. The intrinsic features of NM NMs including ultrafine size, unique optical and electronical properties, especially their easy modifiable surface, offer an opportunity for conjugation with contrast agents, chemotherapeutic agents and physiotherapy agents, to establish innovative theranostic platforms [95]. In this section, we focus on the treatment of hematological malignancies using NM NMs or NM NMs-based curative system in chemotherapy, targeted therapy, and photothermal therapy.
As mentioned for chemotherapy, Pt(II)-based chemotherapeutics are traditional and preferred candidates for the clinical treatment of solid tumors [96]. After the approval of the Food and Drug Administration (FDA) in 1978, Pt chemotherapeutic drugs have evolved initially from cisplatin to carboplatin, lobaplatin and oxaliplatin [97, 98]. The problems of Pt(II)-based chemotherapeutics including poor pharmacology, systemic toxicities, rapid blood clearance, and side effect like nephrotoxicity, neurotoxicity, ototoxicity, and myelosuppression which strongly restrict their clinic efficiency and usable range, needs to be addressed [99]. Hence, major efforts have been made in exploring the new Pt-based drugs in order to eliminate toxic side effects and drug-resistance of tumors. Recently, the effects of small-sized Pt NPs or Pt NCs as anticancer nanomedicine to augment chemotherapeutic efficacy have been investigated [100, 101]. Besides the fluorescent imaging capacity, dual-functional Pt NCs mentioned above showed selective inhibition of hematopoietic K562 and BV173 cancer cells [94]. In contrast to hematopoietic normal cells, these Pt NCs induce a three times higher apoptotic rate in hematopoietic cancer cells. Additionally, immunoblotting was used to confirm the molecular mechanism of Pt NCs’- induced apoptosis and the results suggested that Pt NCs could induce pro-apoptotic protein expression (p53, PUMA, cleaved caspase) in hematopoietic cancer cells, leading to apoptosis in these cells. Some research also reported the apoptosis mechanism of Pt NCs-based chemotherapeutics, that is, high surface-active Pt NCs are eroded to an oxidation state caused by interaction with intracellular acidic organelles (endosomes, lysosomes, etc.) whereby Pt ions damage DNA, giving rise to a synergistic effect of both Pt NCs and Pt ions [52].
In addition to conventional Pt, Au and Ag NMs have also exhibited the induced
apoptosis of hematological malignancies and the possibility for them to be
developed as novel chemotherapeutics for the effective treatment of hematopoietic
system disease, especially AML, lymphoma and multiple myeloma [102, 103, 104]. Kumari
et al. [105] proposed a green nyctanthes arbortristis mediated synthetic
method of Ag NPs with an average size of 22 nm. The different concentrations
(5–50
On account of the unsatisfactory bioavailability and non-specifical
biodistribution of conventional chemotherapeutic drugs, certain
molecular ligands like antibodies, proteins (including their
fragments), nucleic acids (aptamers), and other receptor ligands (peptides, etc.)
impose upon the chemotherapeutics the targeting and selective properties, that is
targeted therapy (Fig. 4) [108]. Targeting agents facilitate the NM NMs to
specifically reorganize the membrane receptors or antigens on target tumor cells
and the type of selective targeting agents greatly determine the physicochemical
properties of conjugates. Hence, NM NMs-ligand conjugates (combination with
specific targeting agents) are a major way to limit the drug distribution
in vivo and target onto the lesion site. Recently, the primary
target site of hematologic malignancies is CD19 despite the increased use
frequency of CD22 and active agents targeting B cell maturation antigen (BCMA)
[109, 110, 111]. For example, Au nanourchins (GNUs) with core diameter at 101
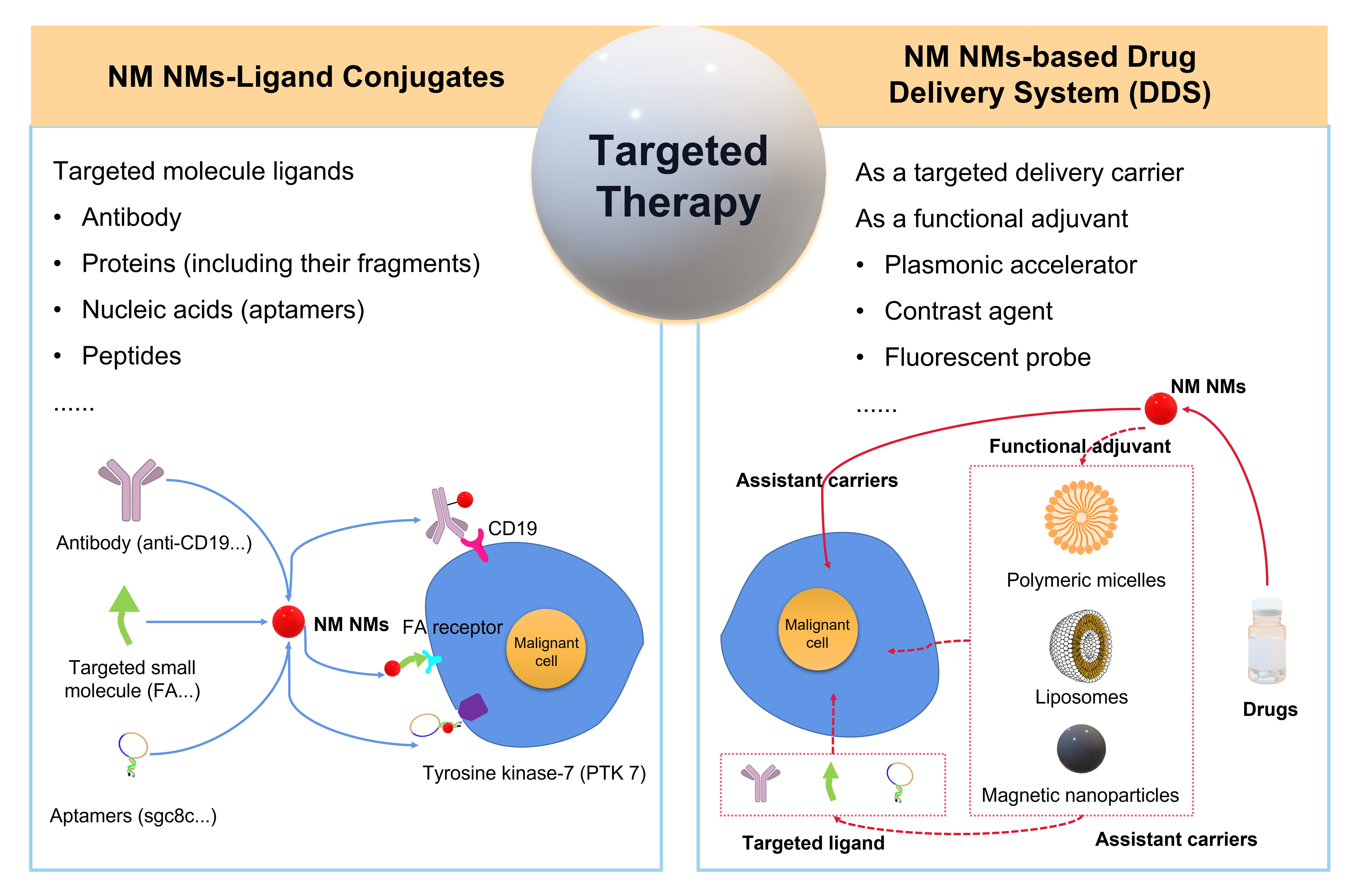 Fig. 4.
Fig. 4.The schematic targeted therapy of hematologic malignancies using NM NMs to construct the NM NMs-ligand conjugates or NM NMs-based drug delivery system (DDS).
Generally, nanomedicines have a tendency to extravasate into solid tumor tissue via permeability and retention (EPR) effects, whereas, vascular phase and diffuse localization of hematologic malignancies make the EPR effect less efficient [115]. NM NMs with outstanding chemicophysical and biological properties can be used as the targeted delivery carriers or functional adjuvants in combination with other carriers like liposomes, biocompatible and degradable polymers, magnetic nanocarrier [40]. The establishment of NM NMs-based drug delivery systems (DDS) for the development of targeted therapy has become an innovative breakthrough in the traditional treatment of refractory cancers especially hematopoietic diseases (Fig. 4). Patra et al. [116] proposed a potential Au-Vel-FA DDS including velcade (Vel) as an anti-cancer drug, folic acid (FA) as a targeting agent and Au NP as a delivery vehicle. Au-Vel-FA was capable of inducing apoptosis against both multiple myeloma U266 and RPMI cells on the premise of maintaining the functional activity of velcade. This form of DDS could be deemed as an equally effective alternative to classical chemotherapeutics and capable of being extended to other malignancies. A triple DDS called DNA-nanosilver-berberine was constructed employing DNA as a carrier Ag NPs as a plasmonic accelerator, and berberine as an effective drug for leukemic cancer [117]. DNA-nanosilver-berberine exerted high toxicity against CCRF-CEM cells inducing apoptosis of cells through increased ROS production and executive caspase 3/7 activation. Ag NPs accelerated the electronic transitions of Berberine and adsorbed the high energy emission absorbent in the integration with penetrative light radiation for deeper localized tumors [118].
In a word, either NM NMs-ligand conjugates or NM NMs-based DDS for targeted therapy not only protect drugs against the external environment during the transportation towards the target site, but also increase the blood circulation time and surface multi-functionality. Most important is that the long circulation properties will increase the possibility of medicable drugs encountering malignant cells in peripheral blood which is one of the main target sites in hematologic malignancies [115]. High efficiency, specific selectivity and unique applicability of targeted therapy makes it advantageous for the treatment of hematologic malignancies, however, it is regrettable that targeted therapy has not shown a survival advantage in stage IV disease for many randomized trials, despite its significantly improving patient survival and quality of life [119].
As a form of photodynamic therapy (PDT), photothermal therapy (PTT) uses heat created by electromagnetic radiation to eliminate or ablate tumor cells [120, 121]. Light-responsive materials can be activated by light or near infrared (NIR) wavelength radiation, resulting in high affinity for cancer cells through hyperthermia [122]. PTT manifests a prominent role in the treatment of cancer based on its non-invasive, non-contact and low cytotoxic properties. Au NPs are preferred in the photo-based nanomedicine because of specific optical features like SPR [123, 124]. If Au NPs enter into tumors, they trend to aggregate at the local tumor sites, and absorb energy via a light (wavelength of 700–980 nm/1000–1400 nm) irradiation to generate heat which can transfer to tumors without damaging normal cells (Fig. 5A) [121, 125]. The first attempt for the photothermal therapy employing Au NPs in vitro was proposed by Lapotko et al. [126, 127]. They used laser activated nano-thermolysis for cell elimination technology (LANTCET) to target thermolysis of leukemia cells by means of producing microbubbles near the NPs under laser irradiation [126, 127]. Afterwards, Au nanorod (Au NRs) as one kind of NPs were conjugated with CD33 monoclonal antibodies (Au NRs-CD33) to achieve nano-thermolysis of human acute leukemia cells by a NIR pulsed-laser illumination [128]. The proportion of dead hematopoietic tumor cells went up 3–4 times after the PTT by Au NRs-CD33. Wang’s group creatively combined both PDT and PTT approaches by constructing an aptamer switch probe (ASP) connected chlorin e6 (Ce6) photosensitizer onto the surface of Au NRs [129]. ASP was formed as the initial sgc8 leukemia aptamer (OFF state) and polyethylene glycol (PEG) linked sgc8 (ON state) to turn on/off the fluorescence of the photosensitizer, leading to PDT controlled by singlet oxygen generation (SOG) as stated in Fig. 5B. Au NRs with a length below 100 nm (Fig. 5C) play a role as a PTT agent by their plasmon resonance absorption (LPRA) in the NIR region. Positive CCRF-CEM (acute lymphoblastic leukemia T-cells) and negative control Ramos cells (acute lymphoblastic leukemia B-cells) were selected to examine the efficiency of PDT/PTT and the result revealed that this multimodal therapy could efficiently kill the CCRF-CEM (cell viability = 39%) after 812 nm NIR laser irradiation for 10 min compared to nontarget Ramos cells (cell viability = 88%) as shown in Fig. 5D,E [129].
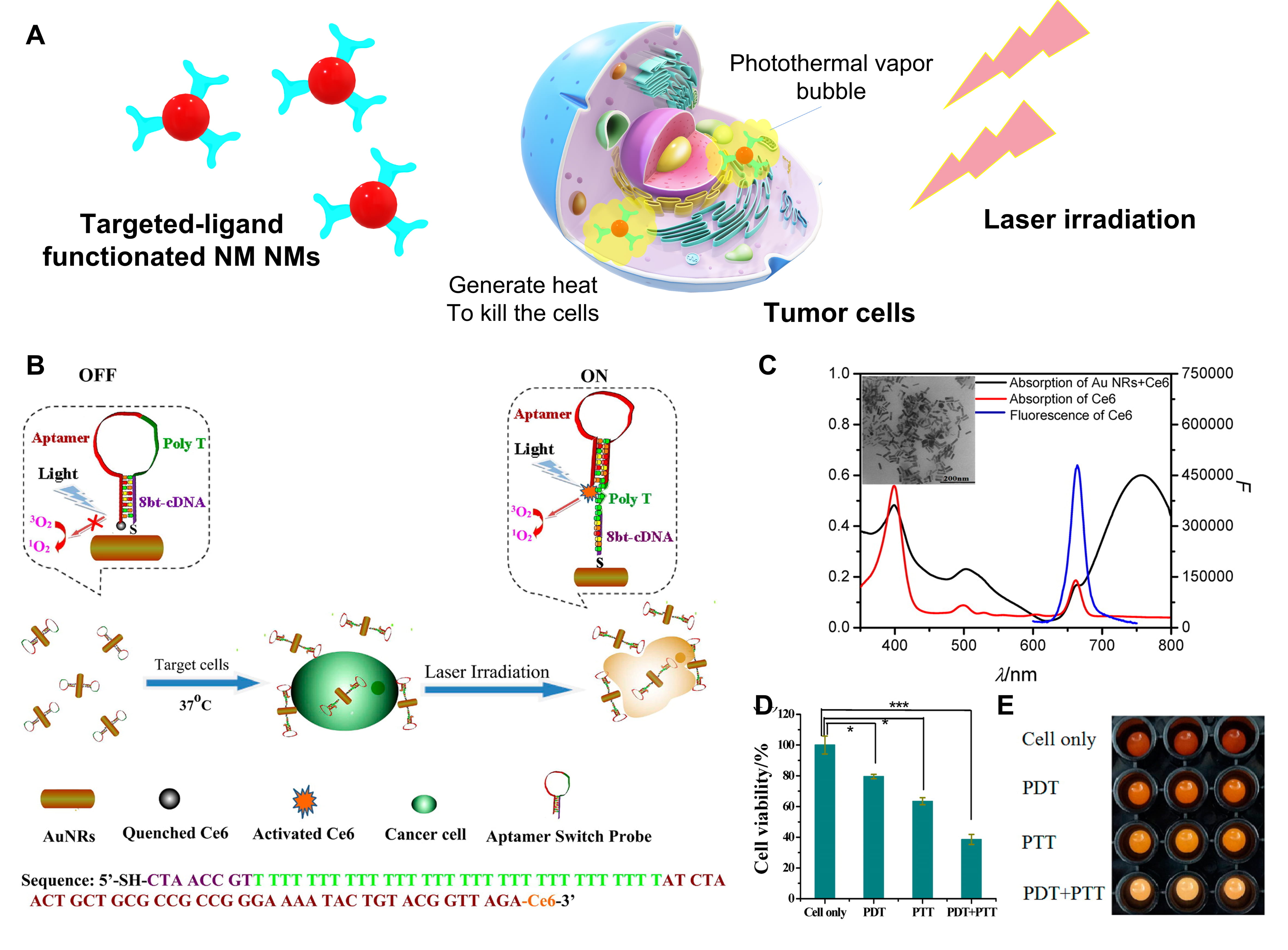 Fig. 5.
Fig. 5.The photothermal therapy of hematological malignancies using
metal nanomaterials. (A) Schematic representation of NM NMs-based photothermal
therapy for tumors. (B) Scheme of the construction of an aptamer switch probe
(ASP) and photosensitizer Ce6 modified the gold nanorods (Au NRs) to form the
ASP-photosensitizer-Au NRs complex for photothermal therapy and photodynamic
therapy. (C) Absorption spectra of Au NRs-Ce6 complex and free Ce6, emission
spectrum of free Ce6. Inset picture is the transmission electron microscope (TEM)
image of Au NRs. (D) Cell viability data and imaging (E) of CCRF-CEM cells
incubated with ASP-photosensitizer–Au NRs without light irradiation (cells
only), under white light irradiation (PDT), under 812 nm laser irradiation (PTT),
and PDT + PTT, respectively. Cells, 200 k/sample; probes, 0.2 nM. p
values were calculated by Student’s t-test: *p
Although NM NMs have remarkable success in PTT for various cancers, the limitations such as inapplicability to metastasing tumors and irreversible tumor growth caused by hyperthermia need specific attention in the future.
This review summarizes three types of hematological malignancies and current advances in their diagnosis and treatment. Considerable effort has been made on the investigation of NM NMs especially Au, Ag, Pt NMs as sensitive probes and safe contrast agents to achieve the detection and cellular imaging of hematologic malignancies due to their peculiar physicochemical properties including LSPR, SERS, and size-dependent fluorescence, as well as relatively low cytotoxicity. Moreover, the intrinsic features of NM NMs such as ultrafine size, unique optical and electronical properties, especially the easy modifiable surface have the ability of integrating these with other contrast agents, chemotherapeutic agents, targeted agents and physiotherapy agents to set up innovative chemotherapeutic, targeted and photothermal therapeutic platforms. In spite of the diagnostic and therapeutic effectiveness of NM NMs, several limitations and imperfections of NM NMs remain to be addressed: (1) the accuracy of the NM NMs-based probes need to be further promoted even at trace levels; (2) exploiting the high fluorescent NM NMs-based biomarker in the NIR range in order to eliminate the interference of autofluorescence and improve the biological imaging effect; (3) NM NMs-based nanomedicine is urgently required to solve the problems of short half-lives, lower bioavailability, and resistance to drugs; (4) new theranostic strategies need to be developed not only limited to traditional chemoradiotherapy, but also extended to immunotherapy, targeted therapy and integrated multi-therapy.
XH proposed the outline of this review and wrote the manuscript. HMM, XD, ZL, XS collected the related literatures and revised the manuscript. ZX, LZ, TL and HL put forward constructive opinions on this review and participated in the writing of the paper. All authors contributed to the article and approved the submitted version.
Not applicable.
Xin Huang gratefully acknowledges the support from “The 2021 Scientific Research and Entrepreneurial Start-Ups Foundation for the Returned Overseas Chinese Scholars, Henan Province”, “The 2018 Backbone Teachers of Zhongyuan University of Technology”, “Zhengzhou Key Laboratory of Green Dyeing and Finishing Technology”, “Henan Collaborative Innovation Centre of Textile and Garment Industry” and “Zhengzhou Key Laboratory of Green Dyeing and Finishing Technology” for their assistance.
This work was funded by National Natural Science Foundation of China (Grant No. 21807121), Henan Provincial Science and Technology Research Project (Grant No. 212102310197), Henan Province Foundation for University Key Teacher (Grant No. 2021GGJS107).
The authors declare no conflict of interest.
