TNF-related apoptosis-inducing ligand (TRAIL/Apo2L), a member of cytokine family, is known to selectively induce apoptosis in cancer cells. However, developing resistance to TRAIL is a major obstacle in cancer therapy. In this study, the in vitro effect of Teucrium alopecurus (TA) essential oil on inhibition of cancer cell growth and enhancing TRAIL-induced apoptosis were investigated in colon cancer cells. Untreated tumor cell lines are used as controls. TA induced cell death and increased the anticancer effects of TRAIL as observed by cell toxicity, live/dead assay, cleavage of caspases and PARP. Furthermore, the mechanism of anticancer potentiating effect of TA was found to be linked with the upregulation of death receptors (DRs) and reduced expression of TRAIL decoy receptors (DcRs). TA also down-regulated antiapoptotic proteins and induced p53 in colon cancer cells. In addition, we observed upregulation of MAPK signalling pathway (p38 kinase, JNK, ERK) and increased expression of C/EBP homologous transcription factor (CHOP) and specificity protein 1 (SP1) by TA. These findings demonstrate the potent anticancer effect of bioactive constituents of Teucrium alopecurus essential oil.
Colorectal cancer (CRC) develops slowly via a progressive accumulation of genetic mutations [1] and leads to cancer-related deaths due to therapy resistance [2]. Therefore, cancer chemopreventive agents gained considerable attention. These agents are either components of natural products or their derivatives, which inhibit the initiation and progression of cancer [3]. Over the past quarter century, research on natural compounds have shown that these agents are safe, effective, and have immediate availability [4]. One of the major sources of chemopreventive agents is the large pharmacopeia of traditional medicines [5]. Whole botanicals have potent antitumor activity at clinically meaningful doses [1].
Many bioactive compounds isolated from medicinal plants have been evaluated for their proapoptotic properties [6]. These bioactive compounds function by modulating the process of xenobiotic biotransformation, inhibiting cellular oxidative damage, or by regulating several other molecules in target cells [3]. A variety of natural compounds provided by Mother Nature have shown to exhibit enormous efficacy against colorectal cancer [1] via the most known cell death pathways [7]. Apoptosis induction, important way to inhibit tumor cell proliferation, has been long regarded as one of the most effective strategies in cancer prevention and therapy [6]. Imbalanced regulation of apoptosis has been shown to cause adverse effects that may lead to the various types of cancer including CRC [8].
TRAIL, a cytotoxic protein, induces cell death by binding to its receptors DR4
and/or DR5 expressed on the membrane of cancer cells [9]. It can also bind to the
decoy receptors DcR1 (TRAIL-R3) and DcR2 (TRAIL-R4), but not able to transmit
apoptotic signal to the cells because these decoy receptors lack cytoplasmic
death domain or have truncated death domain [10]. The cancer cells also developed
resistance to TRAIL overtime. The resistance of tumor cells to TRAIL was found to
be due to high expression of antiapoptotic proteins, such as cFLIP, Bcl-xl, XIAP,
Bcl-2, cIAP-1 and cIAP-2, autocrine production of IL-4 [11]; activation of
NF-
Teucrium alopecurus (TA) is an endemic species native to the Tunisia [17]. In southwest of Tunisia, areal part of this species has been used as folk medicine against variety of diseases for many years alone or in combination with other medicinal plants. TA species is mostly perennial but some species are annuals. The areal parts of this species is used as flavoring agent because of its aromatic taste. In addition, it has an anti-inflammatory activity. It has been found that as a folk medicine, many people apply the powdered form of this species on the external inflamed area to reduce swelling and pain. However, experimental and clinical evidences based medicinal use of this plant has not been well described. This is the first study showing biological, particularly anticancer, activity of T. alopecurus.
This research focuses on the cell death induced by oily fraction of Teucrium alopecurus species and enhancement in antitumor efficacy of TRAIL/Apo2L through increased expression of death receptors, and suppression of antiapoptotic proteins expression. The mechanisms underlying the antitumor potential of the essential oil of interest and whether this latter is able to sensitize CRC to TRAIL-induced apoptosis will be discussed in the following study.
TA was collected from Orbata Mountain, in the city of Gafsa, Tunisia
(coordinates: N 34
Stock solutions of Oily fraction of Teucrium alopecurus leaves (100
The human carcinoma cell lines HCT116 (human colon cancer cells), SCC4 (human squamous cell carcinoma), MCF-7 (breast adenocarcinoma), KBM-5 (human chronic leukemic cells), HL-60 (human promyelocytic leukemia), U266 (human multiple myeloma), MIA PaCa-2 (human pancreatic carcinoma cell line), Panc28 (human pancreatic carcinoma) were obtained from the American Type Culture Collection (Manassas, VA, USA). U266 were cultured in RPMI1640; HCT116, Panc-28, MCF-7, MIA PaCa-2 and SCC4 were cultured in DMEM with 10% fetal bovine serum, 100 U penicillin, and 100 U streptomycin; and KBM-5 cells were cultured in IMDM with 15% FBS and penicillin-streptomycin.
Cytotoxicity was investigated by MTT assay. Briefly, colon cancer cells (5
Tumor cells (2
Clonogenic assay is an in vitro cell survival test based on the ability of a single cell to grow into a colony. Colon cancer cells (500 cells) were seeded in 6-well plates and incubated overnight to allow attachment. The following day, the cells were treated with 200 pg/mL TA, TA + TRAIL, 25 ng/mL TRAIL in triplicate for 24 h. The next day, the medium was changed, and the cells were incubated for 9 days to form colonies. Medium was replaced after 4 days. At the end of the ninth day, medium was removed, cells was washed with PBS, fixed cells and incubated for 20 min. Cells were incubated with 0.5% crystal violet dye for 30 min and washed twice with distilled water, and blue colonies were counted.
Cell extracts were prepared in lysis buffer (20 mM, Tris (pH 7.4), 2 Mm EDTA (pH
8.0), 250 Mm NaCl, 0.01
Then, blots were incubated with secondary antibodies (1 : 5000) diluted in 5% skimmed milk for 1 h, and signals were detected by enhanced chemiluminescence reagent (GE Healthcare, Piscataway, NJ, USA).
Colon cancer cells (1
Data presented are the mean
The FT-IR spectral analyses of the TA essential oil are shown in Fig. 1A.
Infrared region starting from 3600 through 3200 cm
 Fig. 1.
Fig. 1.(A) FTIR and (B) GC/MS spectrum of Teucrium alopecurus essential oil.
Aerial parts of Teucrium alopecurus oils possess different chemical
compositions. The compositional analysis of TA by GC/MS has demonstrated that it
is composed of various terpenic compounds, including (+)-Epi-Bicyclo
sesquiphellandrene, d-limonene,
We used the MTT assay to examine whether TA has the ability to enhance TRAIL-induced cytotoxicity. TA alone increased cytotoxicity of HCT116 cells dependent on dose. As indicated in Fig. 2A, the viability of human colon cancer cells was decreased by 30% and 37% at 100 pg/mL of TA and 25 ng/mL of TRAIL, respectively, however, combination treatment with both TA (100 pg/mL) and TRAIL induces a much higher level (~80%) of cytotoxicity. Pretreatment with TA increased TRAIL-induced growth inhibition.
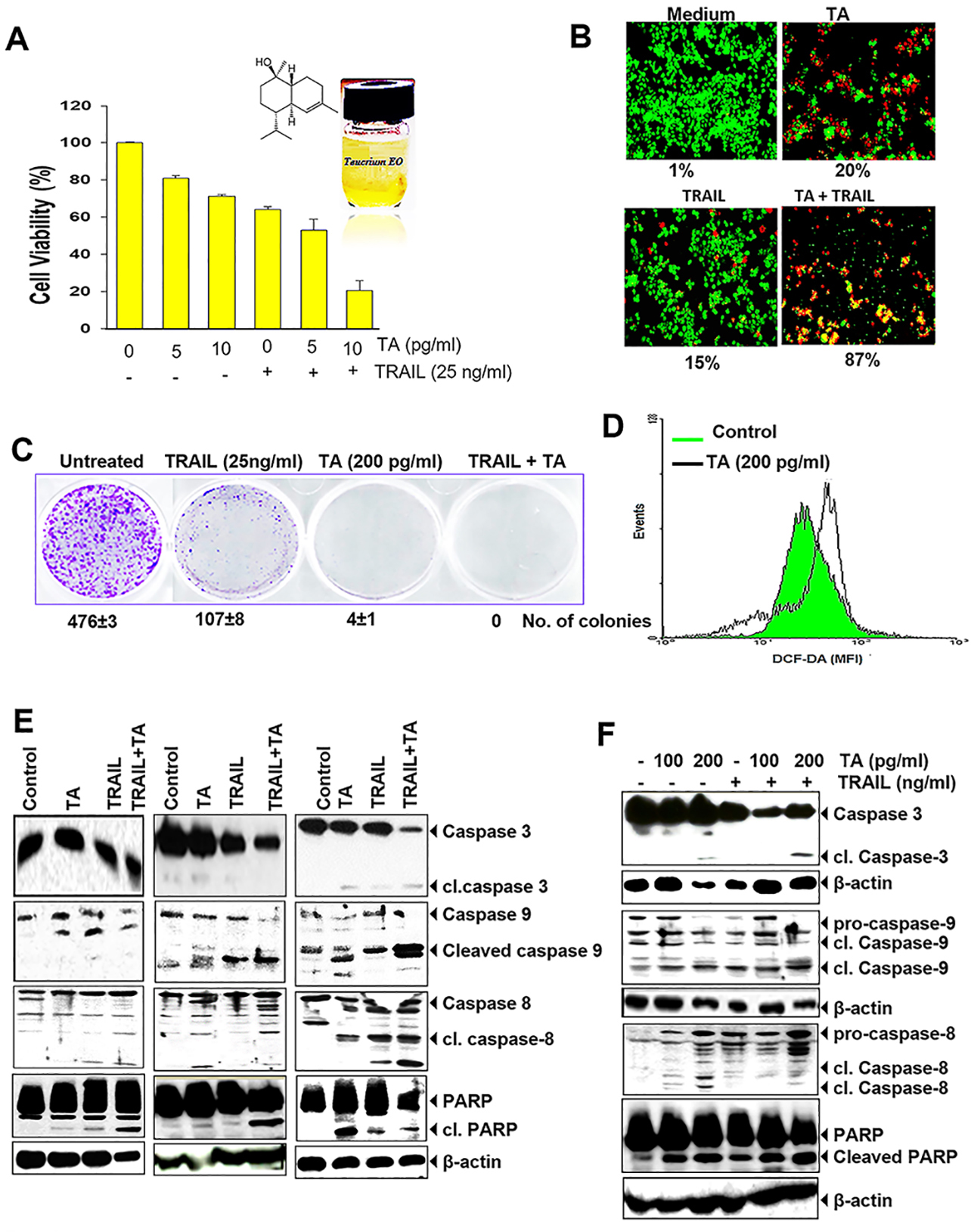 Fig. 2.
Fig. 2.Teucrium alopecurus enhances colon cancer cells to
TRAIL-induced cell death. (A) TA enhances TRAIL-induced cytotoxicity. In 96-well
plates, HCT116 (5,000 cells) were pretreated with two doses of TA (0.005 and 0.01
To assess cell death induced by TRAIL with and without TA, we also used the Live/Dead assay. As shown in Fig. 2B, TA and TRAIL showed modest cell death (20% and 15% respectively). However, when TA and TRAIL were given in combination, a dramatic increase in cell death (87%) was observed. This finding indicates apoptosis potentiating effect of TA.
We also investigated whether TA increases TRAIL-induced suppression of colony-forming ability of HCT116 cells. As shown in Fig. 2C, we found that TA alone represses colony formation. Treatment with TRAIL also moderately suppressed the number of colonies. However, in wells cotreated with TA and TRAIL, we noted almost complete suppression of colony formation.
Since most of the anticancer drugs induce apoptosis through reactive oxygen species (ROS) production, therefore we determined whether TA also generates ROS. We found that TA was able to generate ROS in HCT116 cells. We found a significant production of ROS at 200 pg/mL concentration of TA (MFI 142.4) compared to the control (MFI 114.2) in colorectal cancer cells (Fig. 2D).
To assess whether cotreatment of HCT116 with oily fractions of TA and TRAIL increases apoptosis through cleavage of caspases and PARP dependent on dose- and -time (Fig. 2E,F), we used HCT116 cells. Caspase-8 and -9 activated through extrinsic and intrinsic apoptotic pathway, which further activate effector caspase-3 and then induces PARP cleavage [18]. Administration of TA alone at 100 pg/mL induces little changes in cleaved caspases and PARP levels. Exposure to TRAIL alone induced moderate level of cleaved PARP and caspases (caspase-8, -9 and -3), whereas increased activation of caspases and PARP cleavage were clearly observed in combination of TA (Fig. 2E). When two different doses of TA (100 pg/mL or 200 pg/mL) used alone or in combination with TRAIL (25 ng/mL), both doses increased the apoptotic effect of TRAIL by activating caspases and cleaving PARP (Fig. 2F). Taken together, these results indicate that TA increases TRAIL-induced cell death in colon cancer cells.
To investigate whether TA up-regulates the expression of TRAIL receptors, HCT116 cells was used and treated with TA and found that it increased both DR4 and DR5 receptors dependent on dose- (Fig. 3A) and time (Fig. 3B). Increased DRs expression were detected even at 50 pg/mL of TA. Furthermore, it was observed that TA suppressed the expression of decoy receptors DcR1 and DcR2 (Fig. 3A,B). Our findings indicate that TA upregulates the receptors for TRAIL as well as abolishes the expression of decoy receptors for induction of cell death.
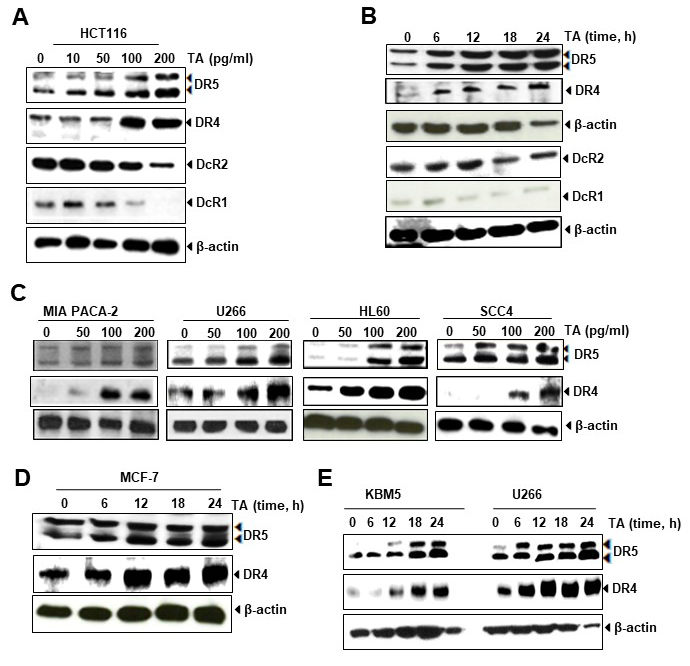 Fig. 3.
Fig. 3.Upregulation of death receptors and down-regulation of Decoy receptors by TA in colon cancer cells. (A) TA induces DRs expression and down-regulates DcRs in HCT116 cancer cells in dose-dependent manner. (B) TA induces DRs expression and down-regulates DcRs in HCT116 cancer cells in time-dependent manner. (C) TA increases expression of DR5 and DR4 in multiple types of cancer cells including MIA Paca-2, U266, HL60 and SCC4 cells. (D) TA induced expression of DR5 and DR4 in breast cancer cells in time dependent manner. (E) TA upregulates expression of DR5 and DR4 dependent on time.
We also investigated whether TA increases DR5 and DR4 expression in HCT116 cells and other tumor cells. As shown in Fig. 3C, induction of TRAIL receptors was noted in MIA Paca-2, U266 cells, HL60, and SCC4 cells. In MCF-7 (Fig. 3D), KBM5 and U266 cells (Fig. 3E), TA increased expression of DR5 and DR4 dependent on time. Taken together, this data indicates that induction of either DR5 or DR4 expression by TA was not specific to the cell-types.
ERK1/2 phosphorylation and JNK have shown to be involved in the upregulation of DR [19]. Moreover, activation of Sp1 [20] and induction of TRAIL-induced apoptosis has been related to the phosphorylation of ERK1/2 [21]. Therefore, we examined whether the TA is able to activate MAPKs such as ERK1/2, JNK, and p38; and inhibit Akt activation. Our results show that TA increased the phosphorylation of ERK1/2 dependent on dose (Fig. 4A) and time (Fig. 4B), and the optimum induction was detected when HCT116 was exposed with 50 pg/mL of this essential oil. It is depicted in this Figure that the oily fractions of Teucrium trigger apoptosis in HCT116 by activating JNK and p38. Overall, these results suggest that TA activates ERK1/2, p38 and JNK molecules dependent on dose and time.
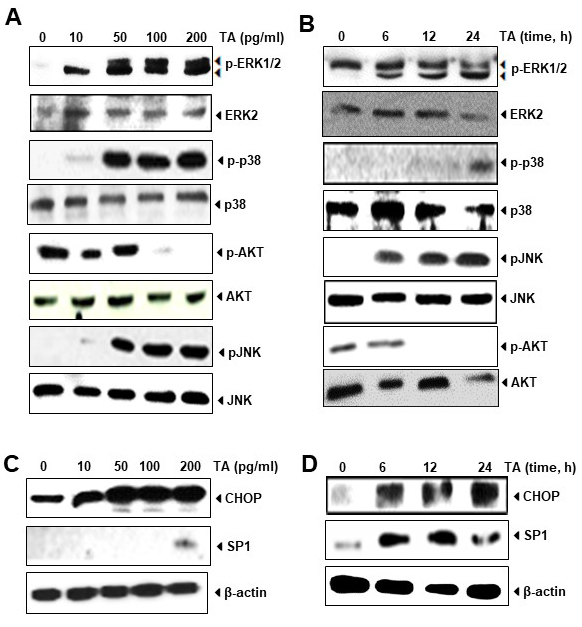 Fig. 4.
Fig. 4.Activation of ERK1/2, p38 and JNK and downregulation of Akt by
TA in colon cancer cells. (A) HCT116 cells was treated with indicated dose of TA
for 6 hours and subjected to western blotting for ERK1/2 (pERK1/2), p38 (pp38),
and JNK (pJNK) expression. Total proteins were used as loading control. (B)
HCT116 cells was treated with TA1 (100 pg/mL) for indicated time and subjected to
western blotting. Total proteins were used as loading control. (C) HCT116 cells
was treated with indicated dose of TA for 6 hours and subjected to western
blotting for CHOP and SP1 proteins.
Sp1 also regulate the expression of DR5 gene by binding on its promoter region [19]. Previously Yoshida [22] also shown that the promoter region of DR5 gene contains binding sites to Sp1. This overexpression induced by Sp1 helps in enhancing TRAIL-mediated apoptosis. Therefore, we determine whether TA also induces the transcription factor Sp1. Our results showed that TA enhances expression of Sp1, which regulates DR5 expression. We also observed induced expression of CHOP by TA in colon cancer cells dependent on dose (Fig. 4C) and time (Fig. 4D). Similar as Sp1 transcription factor, CHOP is also reported to induce apoptosis in cancer cells by upregulating DR5 [23]. The expression of CHOP was induced at as low as 6h after exposure to TA. These observations suggest that induction of DR4 and DR5 by TA is mediated through upregulation of Sp1 and CHOP that further leads to potentiation of TRAIL-induced cell death in cancer cells.
To understand the mechanism by which TA induced apoptosis in HCT116 cells, western blotting has been done. This method used to determine the level of antiapoptotic proteins because Bcl-2 and IAP family proteins play major role in cancer cell growth and proliferation [24, 25]. We noted that TA reduces the expression of antiapoptotic proteins c-myc, cIAPs, Mcl-1, survivin, Bcl-2, metastatic protein ICAM-1 and induces expression of tumor suppressive protein p53 in dose dependent manner (Fig. 5A).
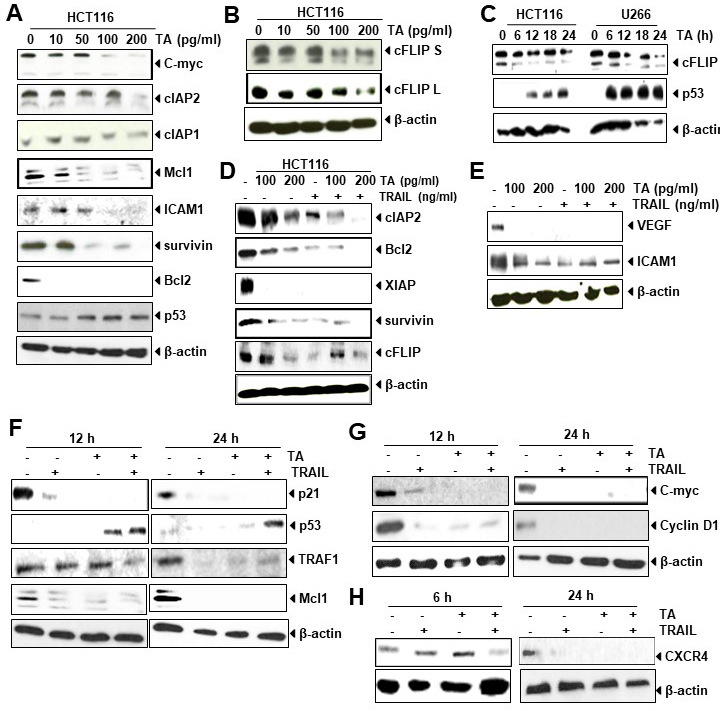 Fig. 5.
Fig. 5.TA suppresses expression of antiapoptotic proteins,
induces p53 and increases effect of TRAIL. (A–B) HCT116 cells were treated with
indicated dose of TA for 24 hours and then western blot was performed against
mentioned proteins.
The overexpression of c-FLIP protein, a caspase-8 inhibitor, is known to
regulate apoptosis mediated by TRAIL in colon cancer cells [26] by conferring
resistance to death receptor [13]. The data obtained from Western blotting
indicated that the administration of TA to colon cancer cells reduced
both c-FLIP isoforms in a time course (Fig. 5B). Besides HCT116 cells, this
protein was also downregulated in U266 cells dependent on time (Fig. 5C).
Overall, our result showed that suppression of c-FLIP
Next, we determined whether TA increases the suppression of antiapoptotic proteins when combined with TRAIL. HCT116 cells was exposed with TA (100–200 pg/mL) alone or in combination with TRAIL and then performed western blotting to determine the effect of this combination. As shown in Fig. 5D, we observed that TA alone dose dependently suppressed the expression cIAP2, Bcl-2, survivin (Fig. 5D), and metastatic protein ICAM1 (Fig. 5E), however, in combination with TRAIL this suppression is significantly increased. The antiapoptotic protein XIAP and angiogenic protein VEGF suppressed at even lower dose of both TA and TRAIL either treated alone or in combination.
We further determined whether TA increases modulation of p21, p53 and antiapoptotic proteins TRAF1 and Mcl1 when combined with TRAIL. For this we treated cells for 12 hours and 24 hours with TA alone or in combination with TRAIL. We found that TA suppressed the expression of p21, TRAF1, and Mcl1 alone or in combination with TRAIL in both 12 and 24 hours of exposure. The effect was more prominent at 24 hours of exposure compared to 12 hours. Similarly we observed increased expression of p53 by TA not by TRAIL at both 12 and 24 hours of exposure (Fig. 5F). Besides these, we also found decreased level of c-Myc, Cyclin D1 (Fig. 5G), and CXCR4 (Fig. 5H) by TA alone or in combination. The effect of TA and/or TRAIL was also more prominent at 24 hours of exposure compared to 12 hours.
Colon cancers has known to develop resistance by adapting molecular changes that allow it survival and unrestricted proliferation under even unfavorable conditions [27]. In the last few decades, importance has been given to biologically active natural products as these compounds have shown generally safe and effective [28, 29]. One of them is Teucrium spp. Study has shown that Teucrium species and their bioactive components exhibit potent anti-inflammatory and cytotoxic activities in vitro models [30].
Numerous chemopreventive compounds exhibit anticancer effects by targeting
apoptosis-inducing signaling pathways in cancer cells [3]. The oily sesquiterpene
alcohol,
TRAIL or Apo2L has been recognized as a potential cancer therapeutic agent clinically [37]. TRAIL induce apoptosis in cancer cells by binding to its agonistic receptors TRAIL-R1 (DR4) and TRAIL-R2 (DR5) [38]. Besides DR4 and DR5, it also binds to the other non-functional receptors (TRID) DcR1 (TRAIL-R3) and DcR2 (TRAIL-R4) that does not lead to transmission of apoptotic signal [37] because they have a truncated death domain [25]. It counteracts death receptors activation [39] and inhibits DR4- and DR5- mediated apoptosis by TRAIL. TRID is beneficial in resisting the potentially harmful effects of constitutively expressed TRAIL in normal growing cells [40]. In this study, we demonstrated that TA sensitize the TRAIL-resistant HCT116 cancer cells by the upregulation of both death receptors DR4 and DR5, which activate the downstream cell death signaling pathways in cancer cells and participate in the TRAIL and other chemotherapeutic agents mediated apoptosis [13]. In this study, we found that TA sensitizes TRAIL/Apo2L to activate DR4 and DR5 expression in human colorectal cancer cell lines, and this upregulation was not tumor type-specific. In addition, hydrophobic fraction of Teucrium was found to decrease the expression of DcR1 and DcR2.
The apoptotic event occurs upon binding of TRAIL to the receptors DR5 or DR4,
which leads to recruitment and activation of caspase 8 through the adaptor
molecule FADD. The activated caspase 8 further activates caspase cascades and
induces apoptosis in cancer cells [10]. DR5 and DR4 genes expression are shown to
be regulated by multiple transcription factors such as p53, CHOP, AP-1, Elk1,
NF-
In response to the DNA damage, activation of p53 occurs that further causes induction of DR5 expression [43]. In addition, p53 regulates the expression of DcR2 [44]. It has been also found that many compounds increase TRAIL-induced cell death in cancer cells through p53-mediated upregulation of DR4 and DR5 [18]. Besides this, upregulation of these death receptors might be due to the induction of CHOP and SP1 [43]. Study has shown that DR5 promoter contains four Sp1-binding regions, which causes upregulation of DR5 gene expression in cells by certain drugs [9]. Our results showed that CHOP and Sp1 proteins expression was activated by oily fractions of TA.
The increased expression of death receptors has been shown to accomplished by activation of MAPK cascade [4]. However, it is known that MAPKs such as JNK, ERK, and p38 kinase involved in the signal transduction of cell proliferation and differentiation as well as in cancer cell death. Essential oils induce apoptosis in cancer cells through the phosphorylation of MAPK [45]. Altogether our results show that TA activated ERK, p38 and JNK and down-regulated Akt (Fig. 6). Essential oils-induced dephosphorylation of Akt may leads to overexpression of p21, which further results in cell death through activation of caspases or causes cell cycle arrest mediated through cyclins [46]. On the basis of the results obtained by Kim [29], it appears that the suppression of Akt activity sensitizes glioma cells to TRAIL-induced cell death through reduction of survivin protein levels. Activation of JNK has also to contribute upregulation of death receptors [12]. The involvement of MAPK kinases in upregulation of death receptors was supported by a study where it was shown that piceatannol-induced upregulation of death receptors requires activation of ERK1/2 and Sp1. This compound activated ERK1/2 and its inhibitor abolished piceatannol-induced expression of TRAIL receptors [47], indicating the importance of ERK1/2 in induction of death receptors.
 Fig. 6.
Fig. 6.Proposed schematic diagram of the apoptotic pathways induced by TA. +: TA enhances TRAIL-induced apoptosis in colon cancer cells (HCT116).
Overexpression of anti-apoptotic proteins including XIAP, c-FLIP, cIAP2, and survivin play major role in the poor prognosis and disease progression in colorectal cancer patients [48]. In our study, TA was found to increase TRAIL-induced cell death of human CRC cell by down-regulating the expression of several antiapoptotic proteins. Thus, the ability of TA to induce apoptosis in CRC may be correlated with its ability to suppress anti-apoptotic proteins and increase upregulation of death receptors. The overexpression of c-FLIP, a negative regulator of death receptor-mediated cell death and inhibitor of caspase-8, has been observed in some cancers, including colon cancer [26]. It has been also shown that high expression level of c-FLIP inhibits binding to FADD protein and causing dissociation of the FADD-caspase 8 complex that results in suppression of extrinsic apoptotic pathway [27] in CRC cells [49, 50]. Moreover, long c-FLIP splice form suppresses the chemotherapy/rTRAIL-induced cell death but the short form of cFLIP has no effect [26]. In addition, bioactive compounds of Teucrium has shown to downmodulate the expression of anti-apoptotic proteins such as survivin, XIAP, c-IAP1, c-IAP2, Bcl-xL and Bcl2, which might play a role in TARIL-induced cell death [18]. Resistance to TRAIL has been also shown to associated with overexpression of anti-apoptotic proteins XIAP and survivin in colon cancer [51] and their inhibition found to be useful sensitization of cancer cells to TRAIL [52]. Upregulation of Bcl2, in turn are responsible for suppression of mitochondria-mediated intrinsic pathway of apoptosis [53]. Our present results indicate that water insoluble fraction of Teucrium species downregulates cell survival proteins. Thus, in CRC, TA induced apoptosis through caspases pathway. Extrinsic pathway of apoptosis requires caspase-8 activation, whereas activation of caspase-9 involve in the extrinsic pathway [18]. Once caspase 8 activated that causes activation of caspase 3 and then convergence of extrinsic and intrinsic pathways of apoptosis takes place [54]. Caspase 9 involve in mitochondria mediated signaling pathway of apoptosis, although this molecule is independent of Bcl2- and death receptor pathways [10]. These data are in agreement with those reported on activation of caspases and PARP cleavage by d-limonene [34]. This study suggests that Teucrium alopecurus essential oil has a therapeutic potential in the treatment of various cancer including cancer of colon. Thus, it potentiates TRAIL to activate death receptors expression in human colorectal cancer cells. However, to validate these findings, further animal and clinical studies are required.
FG and SP contributed to the design of the study. FG developed the initial draft of the manuscript and performed the statistical analyses. All authors analyzed and interpreted the data, critically revised the manuscript, and approved the final draft.
Not applicable.
Drs Wiem Tahri, Imen Dridi equally contributed to this paper. We thank Dr. Bharat B. Aggarwal, Founding Director, Inflammation Research Institute, San Diego, CA; USA and Former Professor of Experimental Therapeutics, Cancer Medicine and Immunology, The University of Texas M. D. Anderson Cancer Center, Houston, TX, USA. The current work was funded by Taif University Researchers Supporting Project number (TURSP 2020/120), Taif university, Taif, Saudi Arabia.
The current work was funded by Taif University Researchers Supporting Project number (TURSP - 2020/120), Taif university, Taif, Saudi Arabia.
Authors have no conflict of interest associated with this research.
BSA, bovine serum albumin; c-FLIP, cellular FLICE inhibitory protein; CHOP = CCAAT/enhancer-binding protein homologous protein; cIAP, cellular inhibitor of apoptosis; DMEM, Dulbecco’s modified Eagle’s medium; DMSO, dimethyl sulfoxide; DRs, death receptors; ERK2, extracellular signal-regulated kinase 2; FBS, fetal bovine serum; FLICE, FADD-like interleukin-1b converting enzyme; IMDM, Iscove’s modified Dulbecco’s medium; JNK, c-Jun N-terminal kinases; PAGE, polyacrylamide gel electrophoresis; PARP, poly (ADP-ribose) polymerase; PBS, phosphate buffered saline; RPMI, Roswell Park Memorial Institute medium; SDS, sodium dodecyl sulphate; TRAIL, TNF-related apoptosis-inducing ligand; XIAP, X-linked IAP.
