† These authors contributed equally.
We evaluated the influence of an antioxidant-rich extract of Filipendula ulmaria L. on anxiety levels induced by nano-sized particles of different calcium phosphates. Rats in experimental groups were administered with either nano-sized hydroxyapatite, tricalcium phosphate, or amorphous calcium phosphate in the presence of Filipendula ulmaria extract. Appropriate behavioral tests were performed to assess anxiety levels, while oxidative status and apoptosis parameters were determined in the hippocampus samples. The applied calcium phosphates increased oxidative stress markers in hippocampal tissue, accompanied by an enhanced pro-apoptotic mechanism. Moreover, the hippocampal immunoreactivity for brain-derived neurotrophic factor and GABAergic-A receptors was significantly lower following calcium phosphate nanoparticles intake. The observed functional and morphological alterations in the rat hippocampus occurred simultaneously with the anxiogenic response estimated in behavioral testing. The neuroprotective effect of Filipendula ulmaria was markedly manifested by the attenuation of oxidative damage induced by amorphous calcium phosphate and enhanced anti-apoptotic action in the rat hippocampus. The increased hippocampal immunoreactivity for brain-derived neurotrophic factor, GABAergic-A receptors and significant anxiolytic-like effects of Filipendula ulmaria may suggest a beneficial role of antioxidant supplementation in preventing anxiogenic response to nano-sized calcium phosphates.
The results obtained in studies performed on cell culture indicate that cell damage was fundamentally caused by calcium phosphate nanoparticle impact on oxidative mechanisms with consequent apoptosis [1]. This has been confirmed by Xu et al. [2] that inhibition of osteoblast activity by hydroxyapatite (HA) nanoparticles was accompanied by apoptosis in vitro through the mitochondrial mechanisms they suggest. The extent of apoptosis was determined by the concentration and size of the HA nanoparticles. The association between cell apoptosis and the size of HA particles was also confirmed by Shi et al. [3] with osteoblast-like cells, while Liu et al. [4] and co-workers concluded that differences in the Ca/P ratio of different CaP phases were among the key factors that determined the level of osteoblast apoptosis that should be considered, besides the particular size, in selecting the type CaPs used for various therapeutic applications. It is usually claimed that oxidative damage may be a substantial part of the pathophysiological mechanism that underlines the numerous toxicities induced by CaP compounds.
Meadowsweet, Filipendula ulmaria (L.) Maxim. – FU is a perennial herb that belongs to the Rosaceae family. Traditionally, the flowers of this plant are used to treat colds and mild rheumatic, renal, and gastric dysfunctions [5]. It is assumed that the aerial parts of FU have numerous medicinal effects: diuretics, antiseptics, antirheumatics, and antacids. Extensive research has shown that FU exhibits strong antioxidant, antimicrobial, cytotoxic, and anti-inflammatory properties [6, 7, 8].
Following the recent trend of administering natural products as antioxidant supplementation in the treatment of various disorders accompanied with oxidative damage, that include confirmation of their neuroprotective role in both pre- and clinical studies [9], there is a growing need for extensive use of calcium phosphates with a bioactive effect due to the confirmed toxic effects in various tissues caused by the use of composites containing nano-CaPs, as well as the fact that the neurotoxicity (including behavioral manifestations).
FU extract (aerial parts of the plant) was chosen in our earlier work [10], exploring the possibility of balancing the redox status altered by different nano-sized CaP compounds. Our previous investigations have confirmed that both pro-depressant effect and cognitive decline were also accompanied by changes in the prefrontal cortex [10]. Here, the intention was to evaluate the potential influence of predefined protocols [10] on other mood disorders, such as the impact on anxiety state level and the different brain structures (hippocampus) responsible for mood regulation. Furthermore, we define morphofunctional alterations in the hippocampus, the region crucially involved in anxiety level regulation, following the chronic intake of nano-sized CaPs with and/or without antioxidant supplementation by FU extract.
The MMA, Serbia, provided Nine-week-old male Wistar albino rats
(weighting 180–220 g). Rats were housed in plexiglass cages (three rats in
each), maintaining the controlled physiological environment (constant temperature
23
Rats were randomly assigned into seven groups (six animals per group). Control group; HA group, that received per os hydroxyapatite (17.8 mg/kg b.w.) for 30 days; HA + FU group, received per os hydroxyapatite (17.8 mg/kg b.w.) and FU extract (100 mg/kg b.w.) for 30 days; tricalcium phosphate (TCP) group, received per os tricalcium phosphate hydrate (11 mg/kg b.w.) for 30 days; TCP + FU group, received per os tricalcium phosphate hydrate (11 mg/kg b.w.) and FU extract (100 mg/kg b.w.) for 30 days; amorphous calcium phosphate (ACP) group, received per os amorphous calcium phosphate (9.65 mg/kg b.w.) for 30 days; and ACP + FU group received per os amorphous calcium phosphate (9.65 mg/kg b.w.) and FU extract (100 mg/kg b.w.) for 30 days.
The characteristics of nano-sized CaPs, purchased from Sigma-Aldrich (Germany), matched those described previously in our work [10]. The Filipendula ulmaria extract preparation and content determination were performed as described in our previous work [6]. Doses of mineral components were equimolar to allow the comparison of individual CaP effects. The applied doses met the lowest HA dose that induced toxic effects in vivoexperiments with nanoparticles [11] and in vitro experiments, which evaluated the concentrations of daily CaPs released from dental composites [12]. To mimic the original route, all CaPs were applied orally. According to our previous results, the plant extract dose was chosen [7], where FU induced significant biological effects. The final concentrations of all estimated compounds were determined following the procedure described in our previous work [10].
The pretreatment and experimental procedures were performed following the European Directive for the Welfare of Laboratory Animals Directive 2010/63/EU, and the GLP principles, as well as the ARRIVE guidelines, following the approval by the Ethical Committee of the Faculty of Medical Sciences, University of Kragujevac, Serbia.
On the day following the end of the pretreatment protocols, rats were accommodated in the testing area for one-hour (around 8 AM). Anxiety level estimation was performed in an open field (OF) and elevated plus maze (EPM) tests. The spontaneous activity in OF maze, followed by EPM, was recorded for five minutes. During 15 minutes between each trial, the mazes were cleaned with water and ethanol (70%) to remove any potentially interfering scents that might affect the test. Video recordings were interpreted with Ethovision software XT 13.0.1220 (Noldus Information Technology, the Netherlands).
OF testing was conducted according to a previously defined algorithm in our lab [8]. The following parameters were calculated: the cumulative duration in the center zone – CDCZ, in seconds, the frequency to the central zone – FCZ, the total distance moved – TDM, in centimeters, the percentage of time moving – %TM, and the wall, free and total number of rearings.
EPM testing was also implemented according to an established standard procedure [13]. The anxiety indicators analyzed from the EPM test were: the cumulative duration in open arms – CDOA, in seconds, the frequency to open arms – FOA, the total distance moved, in cm, the percentage of time moving, the number of walls and free rearings, the number of protected and unprotected head-dippings, and the number of total exploratory activity (TEA) episodes.
After the finalization of behavioral estimation, rats were anesthetized by the combination of ketamine and xylazine (10 and 5 mg/kg, respectively, i.p.) and decapitated. The intact brain hemispheres were promptly and carefully separated and sagittally divided, one for immunohistochemistry and hippocampal tissue dissection (divided for RT PCR and oxidative stress marker analysis).
According to a previously described procedure, the homogenization of
hippocampal tissue and sample preparation (centrifugation at 4000 rpm for 15 min
at 4
Extraction of total RNA from hippocampal samples followed the
manufacturer’s instructions and was performed using PureZOL reagent (Bio-Rad,
USA). iScript Reverse Transcription Mastermix (Bio-Rad, USA) was used for reverse
transcription. Quantitative RT-PCR was obtained with SsoAdvanced Universal SYBR
Green Supermix (Bio-Rad, USA). mRNA-specific primers for Bax, Bcl-2, and
A randomly-selected hemisphere for each rat was fixated in 4%
formaldehyde solution in PBS and paraffin-embedded. Five
SPSS version 20.0 statistical package (IBM SPSS Statistics 20, Chicago,
IL, USA) was used for statistical analysis. The results are expressed as mean
values
Both behavioral tests performed – OF and EPM test, chosen in accordance
with the criteria for an adequate estimation of anxiety. They revealed
significant alterations in anxiety state level following the applied protocols
(Figs. 1,2). The clear anxiogenic response to chronic intake of nano-sized HA and
ACP in OF the significant decrease confirmed the test in cumulative duration and
frequency in the center zone (df = 6, F = 7.033 and 8.445, respectively) when
compared to the control values (Fig. 1A,B, p
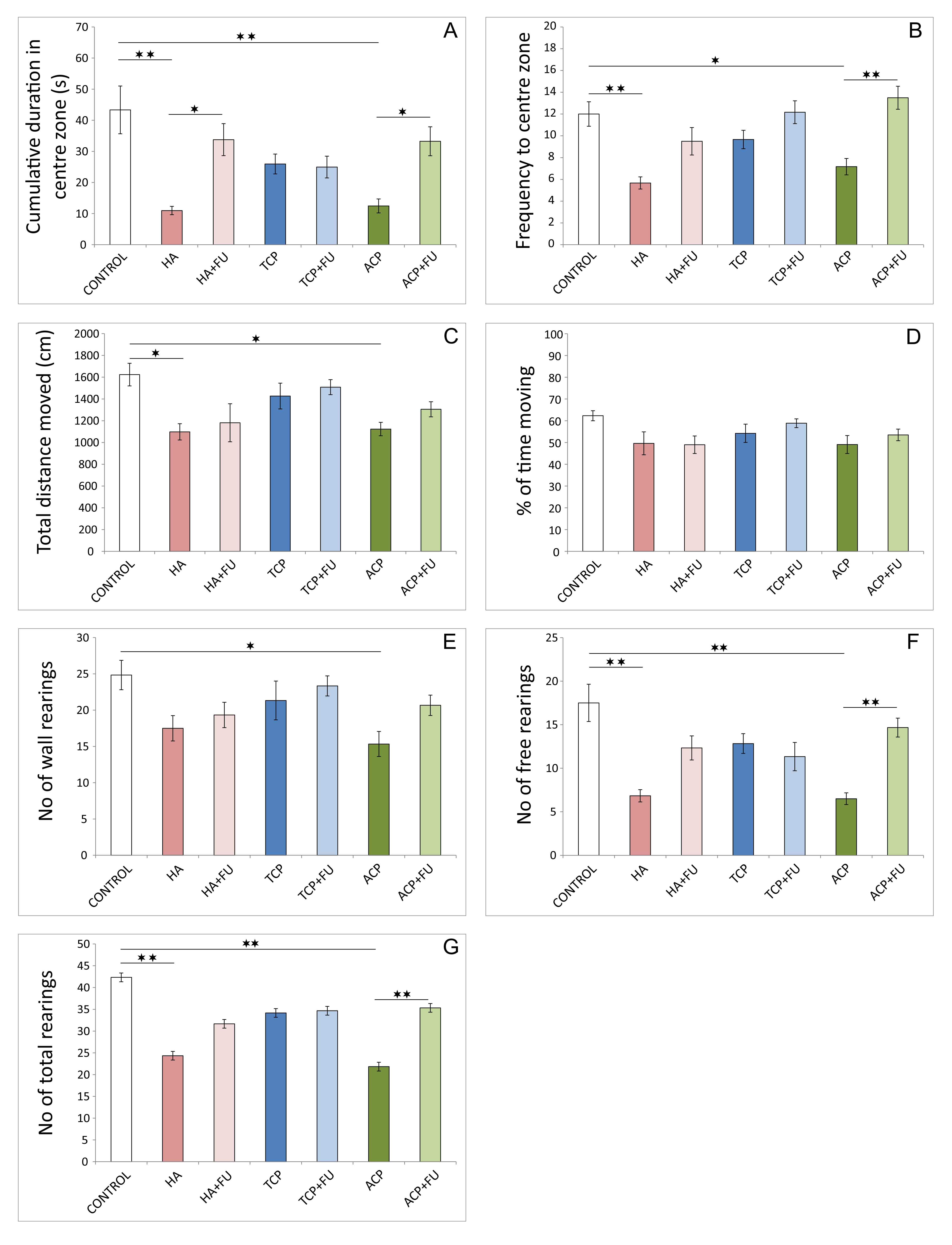 Fig. 1.
Fig. 1.Anxiety indicators were obtained in the open field test (6 per
group). (A) Cumulative duration in the center zone. (B) Frequency to center zone.
(C) Total distance moved. (D) Percentage of time moving. (E) The number of wall
rearings. (F) The number of free rearings. (G) The number of total rearings. HA
– hydroxyapatite; HA + FU – hydroxyapatite and Filipendula ulmaria
extract; TCP – tricalcium phosphate hydrate; TCP + FU – tricalcium phosphate
hydrate and Filipendula ulmaria extract; ACP – amorphous calcium
phosphate; ACP + FU – amorphous calcium phosphate and Filipendula
ulmaria extract. The graph bars present mean
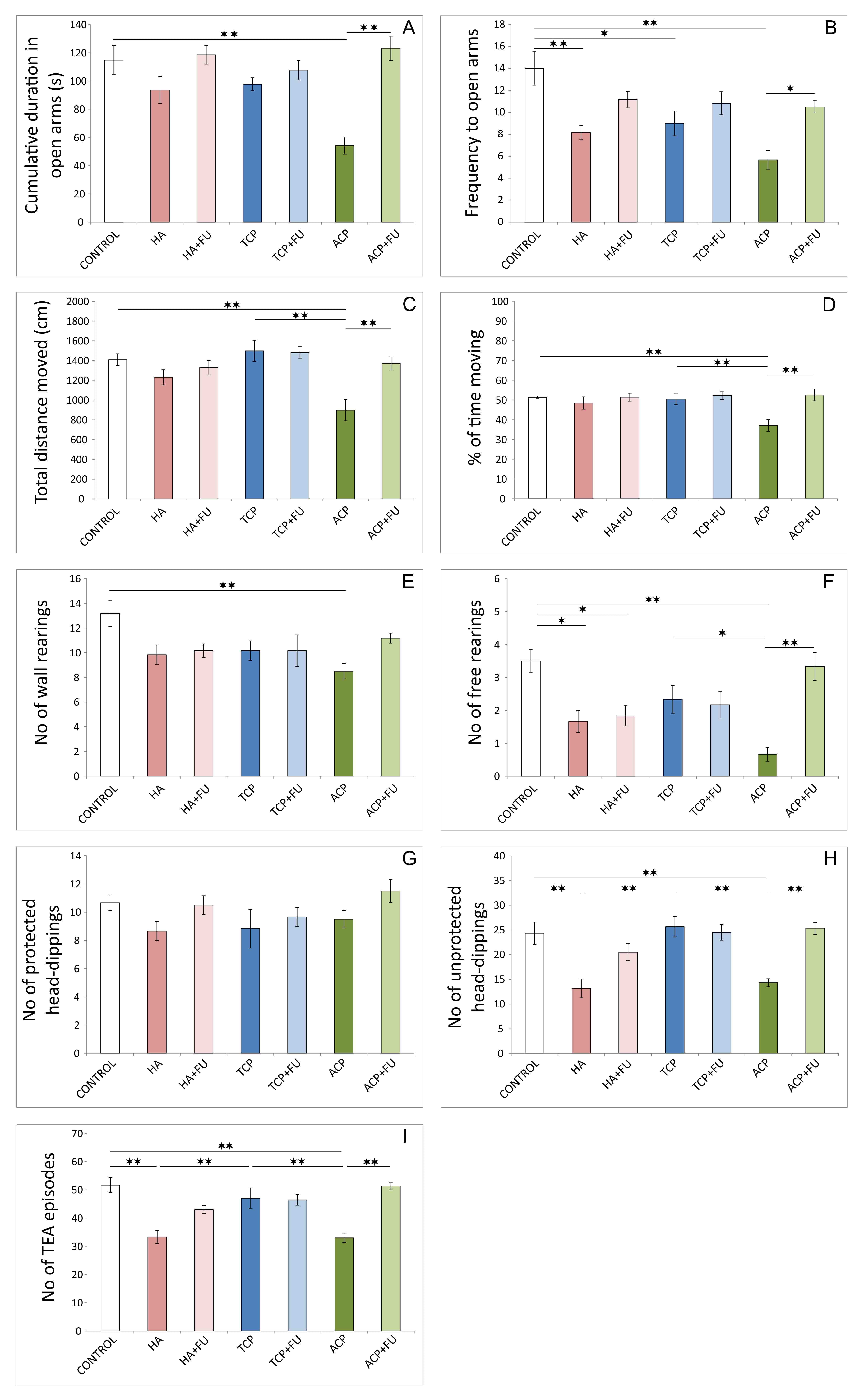 Fig. 2.
Fig. 2.Anxiety indicators were obtained in the elevated plus-maze test
(6 per group). (A) Cumulative duration in open arms. (B) Frequency to open arms.
(C) Total distance moved. (D) Percentage of time moving. (E) The number of free
rearings. (F) The number of wall rearings. (G) The number of protected
head-dippings. (H) The number of unprotected head-dippings. (I) The number of
TEA (total exploratory activity) episodes. HA – hydroxyapatite; HA + FU –
hydroxyapatite and Filipendula ulmaria extract; TCP – tricalcium
phosphate hydrate; TCP + FU – tricalcium phosphate hydrate and
Filipendula ulmaria extract; ACP – amorphous calcium phosphate; ACP + FU –
amorphous calcium phosphate and Filipendula ulmaria extract. The graph
bars present mean
However, antioxidant supplementation successfully prevented this
manifestation of anxiogenic response to HA administration only in an open field.
A significant decline in the number of free rearings in elevated plus-maze test
after FU application was preserved compared to the control (p
The results presented in Fig. 3 confirmed the strong pro-oxidant action
of the applied protocols. The index of lipid peroxidation, expressed as TBARS
(Fig. 3A, F = 9.426), was significantly enhanced by all three nano-sized CaPs
intake (p
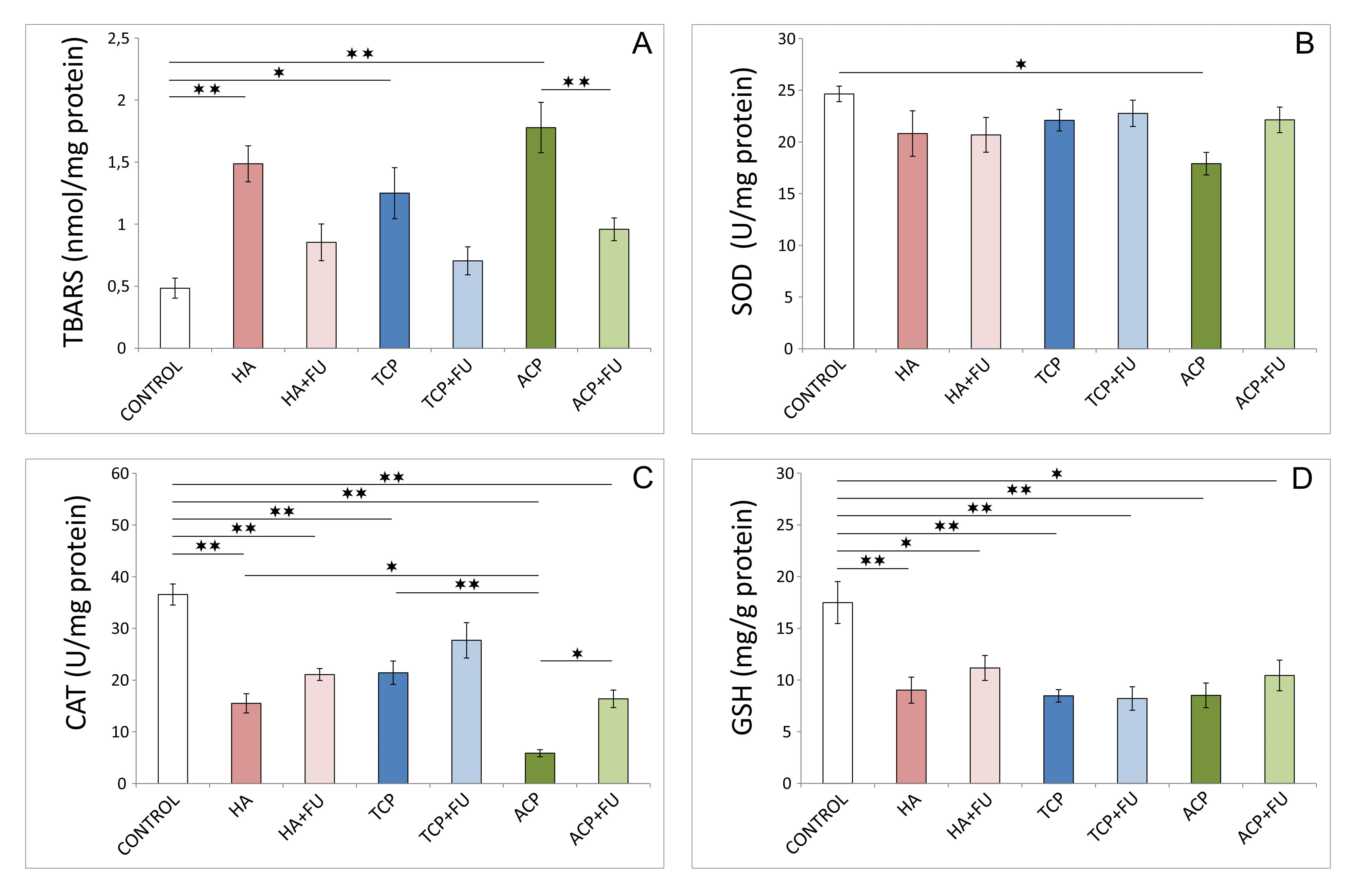 Fig. 3.
Fig. 3.Hippocampal oxidative stress markers (6 per group). (A) Index of
lipid peroxidation (expressed as thiobarbituric acid reactive substances –
TBARS). (B) CAT (catalase) activity. (C) SOD (superoxide dismutase) activity. (D)
GSH (reduced glutathione). HA – hydroxyapatite; HA + FU – hydroxyapatite
and Filipendula ulmaria extract; TCP – tricalcium phosphate hydrate;
TCP + FU – tricalcium phosphate hydrate and Filipendula ulmaria extract;
ACP – amorphous calcium phosphate; ACP + FU – amorphous calcium phosphate
and Filipendula ulmaria extract. The graph bars present mean
As shown in Fig. 4, the relative gene expression of the pro-apoptotic
marker in the hippocampus, estimated using Bax relative gene expression (Fig. 4A),
and the hippocampal anti-apoptotic marker – Bcl-2 (Fig. 4B) was
significantly altered following the chronic administration of CaPs (df = 6, F =
3.896 and 23.364, respectively). The increase in rat hippocampal Bax relative
gene expression following prolonged ACP application was evident compared to
control values (p
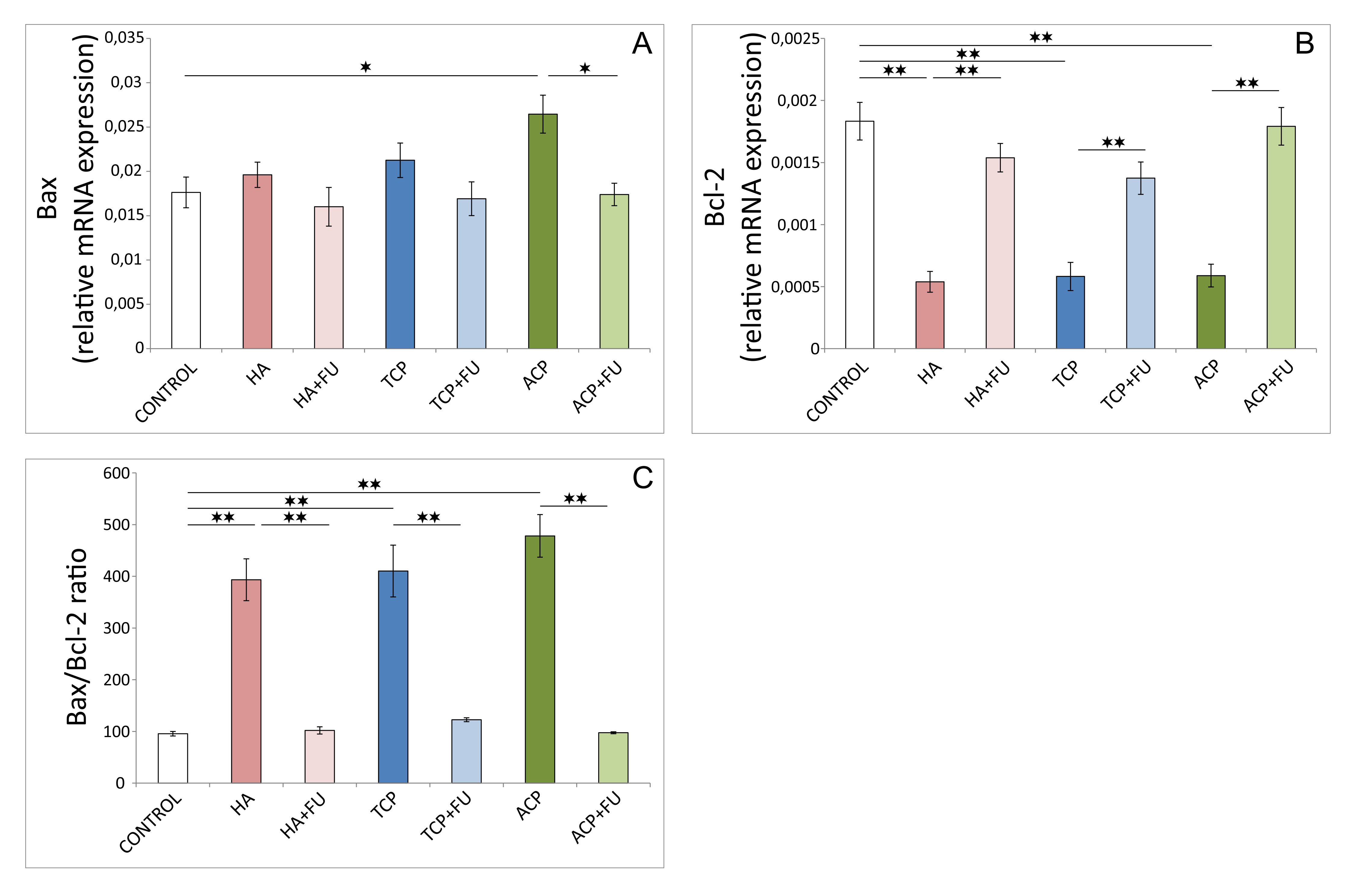 Fig. 4.
Fig. 4.Pro-and anti-apoptotic relative gene expression in the
hippocampus (6 per group). (A) Bax. (B) Bcl-2. (C) Bax/Bcl-2 ratio. HA –
hydroxyapatite; HA + FU – hydroxyapatite and Filipendula ulmaria
extract; TCP – tricalcium phosphate hydrate; TCP + FU – tricalcium phosphate
hydrate and Filipendula ulmaria extract; ACP – amorphous calcium
phosphate; ACP + FU – amorphous calcium phosphate and Filipendula
ulmaria extract. The graph bars present mean
Immunohistochemical analysis also revealed a significant impact of the
pretreatment protocols on the number of brain-derived neurotrophic factor (BDNF)
(Fig. 5) and GABA-AR2S (Fig. 6) immunoreactive cells in the rat hippocampus. The
total number of BDNF immunoreactive cells in the entire hippocampal sections
(Fig. 5D) was significantly reduced by the one-month administration of all CaPs
(F = 79.621, p
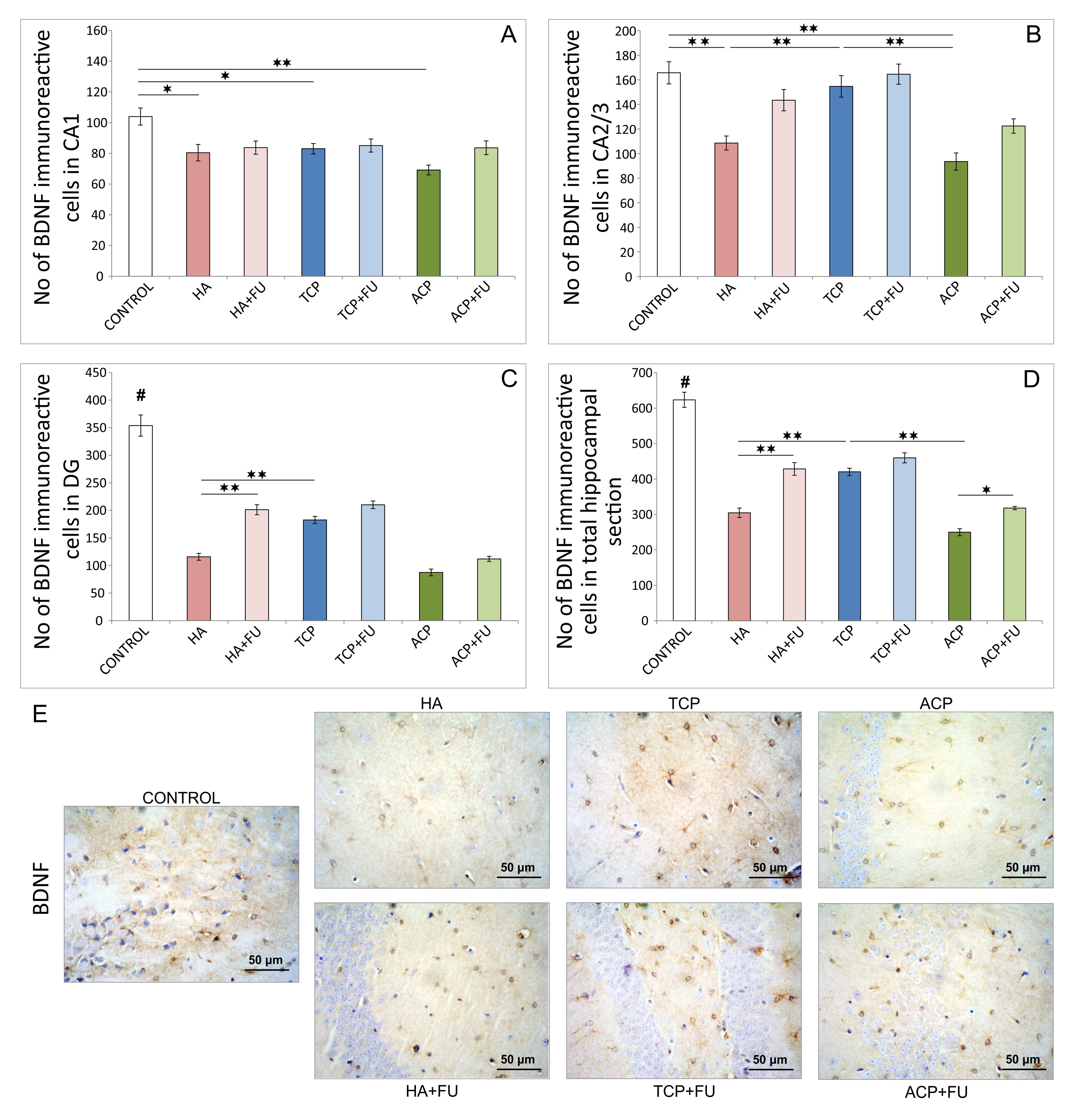 Fig. 5.
Fig. 5.Quantitative immunohistochemical analysis of BDNF-positive cells
in the hippocampus (6 per group) and the distribution in CA1 region (A), CA2/3
region (B), DG (C), and the total section of the hippocampus (D), and (E) the
representative images of BDNF IHC staining (original magnification
As shown in Fig. 6, the applied protocols affected the
number of GABA-AR2S immunoreactive cells in CA1, CA2/3, DG, and total hippocampal
sections (F = 19.839, 13.006, 10.745, and 32.343, respectively). HA and ACP
application resulted in a significant decline of the number of GABA-AR2S
immunoreactive cells in all investigated regions (Fig. 6A–C), as well as
in total hippocampal GABA-AR2S immunoreactivity (Fig. 6D). The same effect was
observed in the TCP group, but the impact of TCP administration was slightly
lower in the CA1 region (p
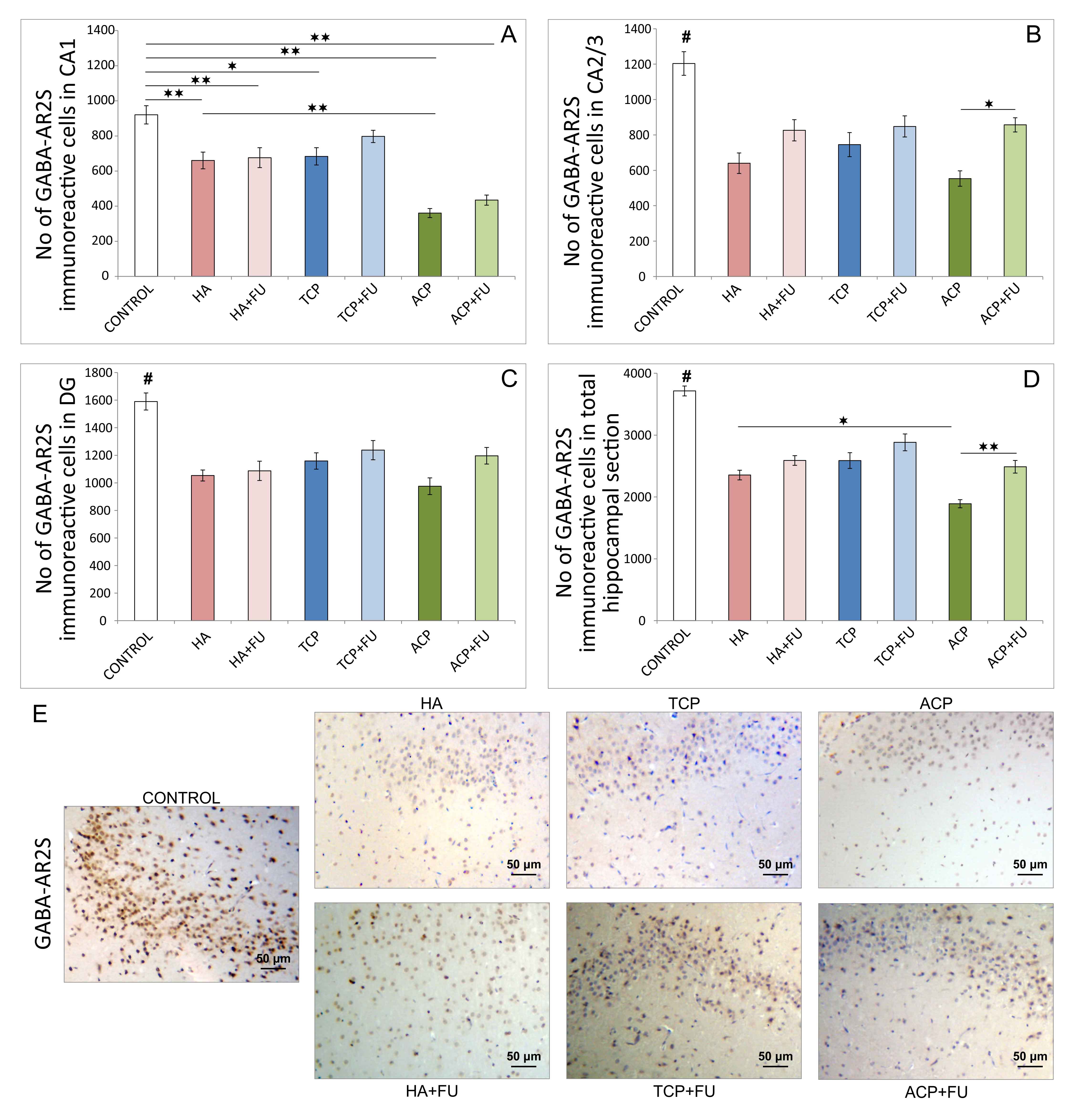 Fig. 6.
Fig. 6.Quantitative immunohistochemical analysis of GABA-AR2S-positive
cells in the hippocampus (6 per group) and the distribution in CA1 region (A),
CA2/3 region (B), DG (C), and the total section of the hippocampus (D), and (E)
the representative images of GABA-AR2S IHC staining (original magnification x20).
HA – hydroxyapatite; HA + FU – hydroxyapatite and Filipendula ulmaria
extract; TCP – tricalcium phosphate hydrate; TCP + FU – tricalcium phosphate
hydrate and Filipendula ulmaria extract; ACP – amorphous calcium
phosphate; ACP + FU – amorphous calcium phosphate and Filipendula
ulmaria extract. The graph bars present mean
The widespread medical use of nanoparticles, including CaPs, is associated with confirmed toxicities in numerous organs and tissues. Further elucidation is therefore required to determine the general mechanism (including oxidative damage) that may compromise their efficiency and safety. Consequently, it seems appropriate to evaluate and promote the prevention of nanoparticle-induced toxicities by antioxidant supplementation. Furthermore, there is a growing need for antioxidant components obtained from natural products. When taken together, this allows the two current medical approaches explored here (the employment of nanomaterials and simultaneous attenuation of their toxicities) to complement each other.
As described above, it is clear that the CaP compounds applied in nanoparticles significantly altered numerous parameters estimated. This is not surprising since literature data indicate different pathways for the entrance of nanoparticles of other substances into the central nervous system, accompanied by neurotoxicity with behavioral manifestations [21]. Also, there is evidence that nano-CaP compounds enter numerous brain regions, including the hippocampus [22], which is the initiating point for this investigation, confirming earlier research that concluded that these compounds induce neurotoxicity with a prodepressant outcome and cognitive impairment [10].
The impact of CaP nanoparticles employed on oxidative stress markers (Fig. 3) is in accordance with the results of previous investigations that analyzed the mechanisms of those nanoparticles’ toxicities. As a postulated mechanism, it has been suggested that nano-HA increased ROS generation, predominantly via hydroxyl radical production [23]. Furthermore, the oxidative imbalance induced by nano-HA has been associated with a decline in cellular antioxidant capacity expressed as a reduction in SOD activity in C6 cell cultures [1]. Results obtained confirmed that antioxidant enzyme activity was more affected by the diminished CAT activity. Interestingly, the values that resume the cellular antioxidant capacity based on antioxidant enzyme activity were not uniformly improved by antioxidant supplementation, while the non-enzymatic antioxidant defense system depletion (expressed by GSH) was not successfully recovered by FU extract.
There is considerable evidence that oxidative damage significantly affects the balance between pro-and anti-apoptotic factors [24]. Following the results for oxidative stress markers obtained, it is not surprising that the applied protocols with nano-sized CaPs potentiated the pro-apoptotic outcome (Fig. 4). Namely, the direct impact of mineral components on the augmentation of pro-apoptotic relative gene expression (Bax) was significant only in the ACP group. The minimization of anti-apoptotic relative gene expression (Bcl-2) was observed for all three mineral components administered. Antioxidant supplementation with FU extract was beneficial using attenuation of pro-apoptotic and potentiating anti-apoptotic relative gene expression. This was confirmed by improving the Bax/Bcl-2 ratio observed following prolonged administration of mineral components.
The results obtained are concordant with previous reports that evaluated the mechanisms of CaP-induced apoptosis. Principally, it seems that both the size and the chemical structure of mineral components significantly affect apoptotic mechanisms in various tissues. In general, metallic nanoparticles have been reported to increase NO release and pro-inflammatory factors such as tumor necrosis factor-alpha by activated microglia in cell culture [25]. Considering the impact of chemical structure on the apoptotic response to CaPs, it should be mentioned that HA nanoparticle administration was associated with an increased p53, down-regulation of Bcl-2, and general DNA damage in rat kidneys [26]. At the same time, nano-ACP can cause apoptosis of the leukemia P388 cells by selectively acting on G1 in the cell cycle [27]. Increased intracellular calcium level (probably due to its release from nanoparticles) has been reported to trigger apoptosis [28]. Additionally, increased intracellular PO43- has been suggested to induce apoptosis through modulation of the mitochondrial membrane potential [29]. Generally, it should be noted that there is still no consensus as to whether CaPs induce apoptosis via caspase-dependent or independent pathways [30].
The pro-apoptotic action of all three mineral components applied accompanied hippocampal BDNF decline (Fig. 5). Although it is well known that BDNF has anti-apoptotic potential in various tissues [31], including CNS [32], it has also been reported that apoptosis itself, especially in the regions responsible for BDNF production, may significantly reduce neurogenesis [33], thus affecting BDNF content. Indeed, the results confirmed that the groups with mineral components intake, with a marked pro-apoptotic outcome, also expressed the lowest hippocampal BDNF immunoreactivity. Furthermore, since BDNF is highly responsible for hippocampal neurogenesis [34], it is not surprising that GABA-AR were also at their lowest levels in the groups (HA and ACP) with evident pro-apoptotic action and down-regulation of hippocampal BDNF (Fig. 6). Our results also agree with a confirmed connection between increased BDNF and up-regulation of the GABAergic system [35].
Finally, the results of behavioral testing performed strongly confirmed an anxiogenic response to the administered mineral components (Figs. 1,2), with the most pronounced effect observed with HA, especially ACP nanoparticles. Increased anxiety following prolonged HA and ACP application may be associated with the previously discussed down-regulation of the hippocampal GABAergic system, expressed using a decline in the number of GABA-AR2S immunoreactive cells in all investigated hippocampal regions. The results obtained for the alterations in anxiety level that correspond to hippocampal GABA-AR expression follow previously reported evidence that GABAergic dysfunction plays one of the key roles in the pathogenesis of various mood disorders [36] and anxiety [37, 38]. Moreover, it should be mentioned that considerable evidence was found for the impact of some of the other parameters evaluated contributing to anxiety level regulation. Therefore, it has been reported that the experimental protocols that resulted in pro-oxidative [39] and pro-apoptotic [13] action in hippocampal tissue significantly enhanced anxiogenic response. At the same time, the down-regulation of hippocampal BDNF was also associated with increased anxiety [40]. Although not evaluated in this study, the additional pathophysiological mechanism for mood alterations could be found in astrocytic impairment in rat hippocampus that affects other transmitters and result in mood deprivation [41].
The results considering the consequences of this antioxidant-rich FU extract usage confirmed that this therapeutic approach might be beneficial in the prevention of the described mood alterations induced by nano-CaPs. At the dose applied, the potentially protective role of FU extract was confirmed by its attenuation of numerous anxiogenic factors such as oxidative damage, up-regulation of anti-apoptotic and down-regulation pro-apoptotic factors well as increased hippocampal BDNF and GABA-AR2S expression. The improvement in anxiety level regulation parameters following antioxidant supplementation observed further confirms the findings of recently published investigations performed in our lab [40].
This research analyzed the possible influence of an antioxidant-rich extract of FU on anxiety level alterations induced by nano-sized particles of different CaPs. As previously noted, this investigation, based on the results obtained in previous work [42], should be followed by a broader approach that includes other nano-CaPs, with a different experimental design (including more prolonged treatment, and finally should explore other brain regions responsible for mood regulation).
The results obtained suggest a growing need for new materials in restorative dentistry should include, besides the estimation of local tissue response, an adequate methodology for the necessary estimation of various potential side effects, including neurotoxicity and its behavioral manifestations. It is worth noting that some differences for individual nano-CaPs were observed for the behavioral manifestations of neurotoxicity. From this perspective, it would appear that TCP was less harmful using anxiety level alteration following the protocols applied herein. Additionally, it seems that antioxidant supplementation may be useful in the prevention and/or treatment of toxicities induced by CaPs.
CaP, calcium phosphate; HA, hydroxyapatite; TCP, tricalcium phosphate; ACP, amorphous calcium phosphate; FU, Filipendula ulmaria; OF, open field; EPM, elevated plus maze; CDCZ, cumulative duration in the center zone; FCZ, frequency to the central zone; TDM, total distance moved; %TM, percentage of time moving; CDOA, cumulative duration in open arms; FOA, frequency to open arms; TEA, total exploratory activity; SOD, superoxide dismutase; CAT, catalase; GSH, glutathione.
Conceptualization—NA, DS, NJ and GR; methodology—NA, DS, NJ and GR; formal analysis and investigation—NA, DS, JSKS, VM, SM, JM, PM, MV, AN, OM-DJ, MZ, NF, VJ, NJ and GR; resources—NA, DS, VJ, NJ and GR; data curation—NA, DS, NJ and GR; writing—original draft preparation—NA, DS, NJ and GR; writing—review and editing—NA, DS, NJ and GR; visualization—NA, DS, NJ and GR. All authors have read and agreed to the published version of the manuscript.
The pre-treatment and experimental procedures were performed following the European Directive for the Welfare of Laboratory Animals Directive 2010/63/EU (ethic code: 01-1377/2021), and the GLP principles, as well as the ARRIVE guidelines, following the approval by the Ethical Committee of the Faculty of Medical Sciences, University of Kragujevac, Serbia.
This work was supported by the Faculty of Medical Sciences, University of Kragujevac, Serbia (JP 01/19), and Ministry of Education, Science, and Technological Development of the Republic of Serbia 451-03-68/2020-14/200107 (Faculty of Engineering, University of Kragujevac), 451-03-68/2020-14/200378 (Institute for Information Technologies Kragujevac, University of Kragujevac) and 451-03-68/2020-14/200122 (Faculty of Science).
This research received no external funding.
The authors declare no conflict of interest.
