† These authors contributed equally.
The disorder of lipid metabolism, especially cholesterol metabolism, can promote Alzheimer’s Disease. Curcumin can ameliorate lipid metabolic disorder in the brain of Alzheimer’s Disease patients, while the mechanism is not clear. APP/PS1 (APPswe/PSEN1dE9) double transgenic mice were divided into dementia, low-dose, and high-dose groups and then fed for six months with different dietary concentrations of curcumin. Morris water maze was used to evaluate the transgenic mice’s special cognitive and memory ability in each group. In contrast, the cholesterol oxidase-colorimetric method was used to measure total serum cholesterol and high-density lipoprotein levels. Immunohistochemistry was used to evaluate the expression of liver X receptor-β, ATP binding cassette A1 and apolipoprotein A1 of the hippocampus and Aβ42 in the brains of transgenic mice. The mRNA and protein expression levels of liver X receptor-β, retinoid X receptor-α and ATP binding cassette A1 were evaluated using qRT-PCR and Western blotting, respectively. Curcumin improved the special cognitive and memory ability of transgenic Alzheimer’s Disease Mice. The total serum cholesterol decreased in Alzheimer’s Disease mice fed the curcumin diet, while the high-density lipoprotein increased. The curcumin diet was associated with reduced expression of Aβ and increased expression of liver X receptor-β, ATP binding cassette A1, and apolipoprotein A1 in the CA1 region of the hippocampus. The mRNA and protein levels of retinoid X receptor-α, liver X receptor-β, and ATP binding cassette A1 were higher in the brains of Alzheimer’s Disease mice fed the curcumin diet. Our results point to the mechanism by which curcumin improves lipid metabolic disorders in Alzheimer’s Disease via the ATP binding cassette A1 transmembrane transport system.
Alzheimer’s disease (AD) is an insidious neurodegenerative disorder. The clinical features of AD are progressive cognitive dysfunction and language and behavioral disorders, social dysfunction, and eventually death [1]. Cholesterol is an essential part of cell membranes and myelinated axons, thereby playing an essential role in maintaining neuronal integrity and functionality [2]. Although the brain is the organ with the highest cholesterol content in humans [3], it cannot absorb cholesterol directly from the blood because of the blood-brain barrier. Cholesterol synthesis in the brain is therefore carried out mainly by astrocytes and neurons [4]. Cholesterol metabolism disorder is a major risk element to cardiovascular disease and may play a role in AD. Several clinical and epidemiological studies have reported a close link between cholesterol metabolism abnormality and AD [5].
ABCA1, a member of the ATP-binding cassette family, plays a pivotal role in
cholesterol transport. The cholesterol transport regulated by ABCA1 also plays an
essential part in the formation of
Curcumin is a special phenolic compound derived from the plant Curcuma longa. It is used clinically as an antioxidant, anti-inflammatory, anti-atherosclerosis, anti-coagulation and anti-tumor agent [10]. The role of curcumin in reducing cholesterol levels is attracting more and more attention. Many researchers have started using curcumin to treat various transgenic animal models for diseases caused by lipid metabolism disorders, such as arteriosclerosis and hypercholesterolemia, with positive results. These studies demonstrated that curcumin could reduce cholesterol levels to maintain body balance [11, 12]. However, the brain cholesterol level is not correlated with the blood cholesterol concentration, and its synthesis mechanism is also different [13]. It is unknown whether curcumin can reduce age-related changes in the brain and gradually increase cholesterol levels in the neurons of AD patients.
This research uses the APP/PS1 transgenic mouse model to observe the impact of different curcumin concentrations on cholesterol content and the LXR/RXR-mediated ABCA1 transmembrane transport system. This original experimental evidence should help to find new treatments and targets for AD.
APP/PSI (APPswe/PSEN1dE9) transgenic mice (Alzheimer’s disease mouse model) and the type of wild C57BL/6J mice (control mice) were supplied through the Institute for Laboratory Animals, Nanjing Biomedical Research Institute, Nanjing University. Total animal experiments were carried out by the guidelines to Care and Use for experimental Laboratory Animals (NIH Publication No. 85–23, revised 1996). They were accepted through the Research Ethics Committee of the Chongqing University Cancer Hospital. Six-month-old APP/PSI double transgenic mice were divided into three groups randomly. They fed different concentrations of curcumin-mixed feeds: the control group was fed a regular diet without curcumin. The Cur-L group was fed 0.16 g/kg of curcumin- (Sigma-Aldrich) mixed, and Cur-H was fed 1.0 g/kg of curcumin-mixed feeds. The experiments were carried out after feeding this diet for six months.
Mice were anesthetized by isoflurane deeply before obtaining tissue samples. A
proportion of the mice was infused with 0.9% normal saline, and the brain
tissues were stored at –80
Mouse anti-ABCA1 and goat anti-LXR-
Specific protocols for the Morris water maze test were described in our previous research [14]. The experiment included a visual platform test (day 1), a hidden platform test (days 2 to 6), and a probe test (day 7).
Liquids were mixed according to instructions for the total cholesterol (CHO enzymatic) test kit and the serum high-density lipoprotein kit (Nanjing Jiancheng Biological Engineering). Samples were then added according to the relevant instructions, and the absorbance was measured for each tube.
The specific protocol for Western blot assay is described previously [14].
Protein from brain tissues was homogenized using a protein extraction reagent
(RIPA:PMSF = 100:1). Protein concentrations were identified through the BCA assay
(Beyotime, China). For Western blot analyses, proteins (35
The brains of mice were fixed with 4% paraformaldehyde, embedded in paraffin,
sliced five
RNA was separated through brain tissues using RNAiso Plus (TaKaRa). PrimeSriptTM
RT reagent Kit (TaKaRa) compound first-strand cDNA based on the
manufacturer’s introduction. The cDNA was further amplified by SYBR Premix Ex
TaqTM II (TaKaRa) in a 20
Statistical analyses were performed using SPSS software (version
17.0, IBM Corp., Chicago, IL, USA). Experimental data are represented by the mean
and standard error of the mean (SEM). Experimental results were compared using
two-way ANOVA, next by multiple comparisons using Bonferroni post-test.
p
MWM was used to evaluate the hippocampal-dependent spatial learning and memory
ability of the APP/PS1 double transgenic mouse model to AD. During the learning
phase (Fig. 1A), the mice fed low-dose Curcumin (Cur-L group) demonstrated
significantly shorter times to discover the evacuation platform (shorter escape
latencies) compared to mice from the control group (p
 Fig. 1.
Fig. 1.Curcumin improved cognitive function of APP/PS1 double
transgenic mice. (A) The escape latency during the 5-day MWM testes. (B) The
number of crossing the target on the last day of the MWM test (n = 5, two-way
repeated-measures ANOVA, Tukey’s test, #p
Immunohistochemistry was used to detect A
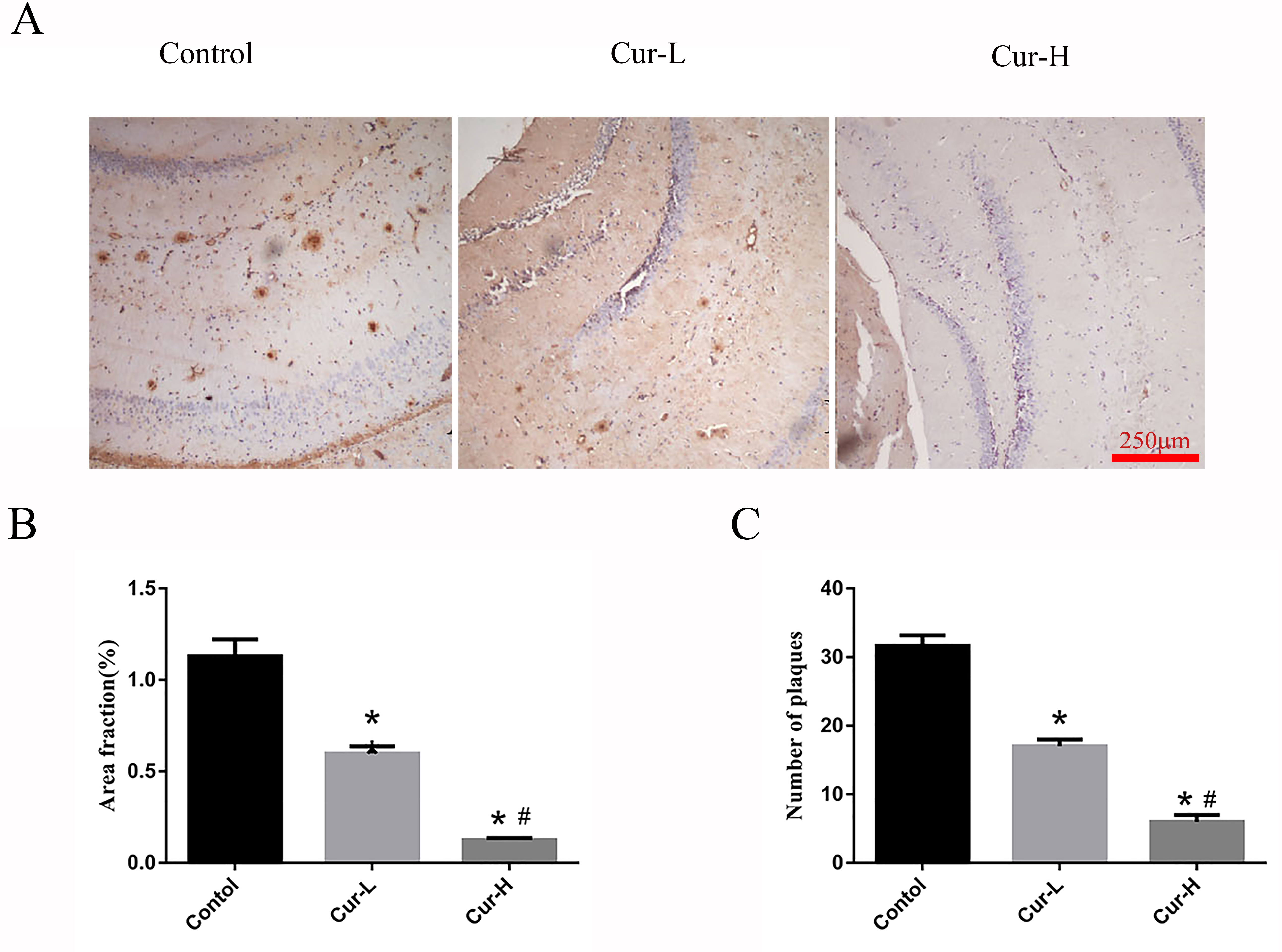 Fig. 2.
Fig. 2.Curcumin reduced A
Using enzymatic detection, the serum TC levels for each group were found to be
different (Fig. 3). Serum TC levels in the Cur-L group were dramatically lower
than in the control group (p
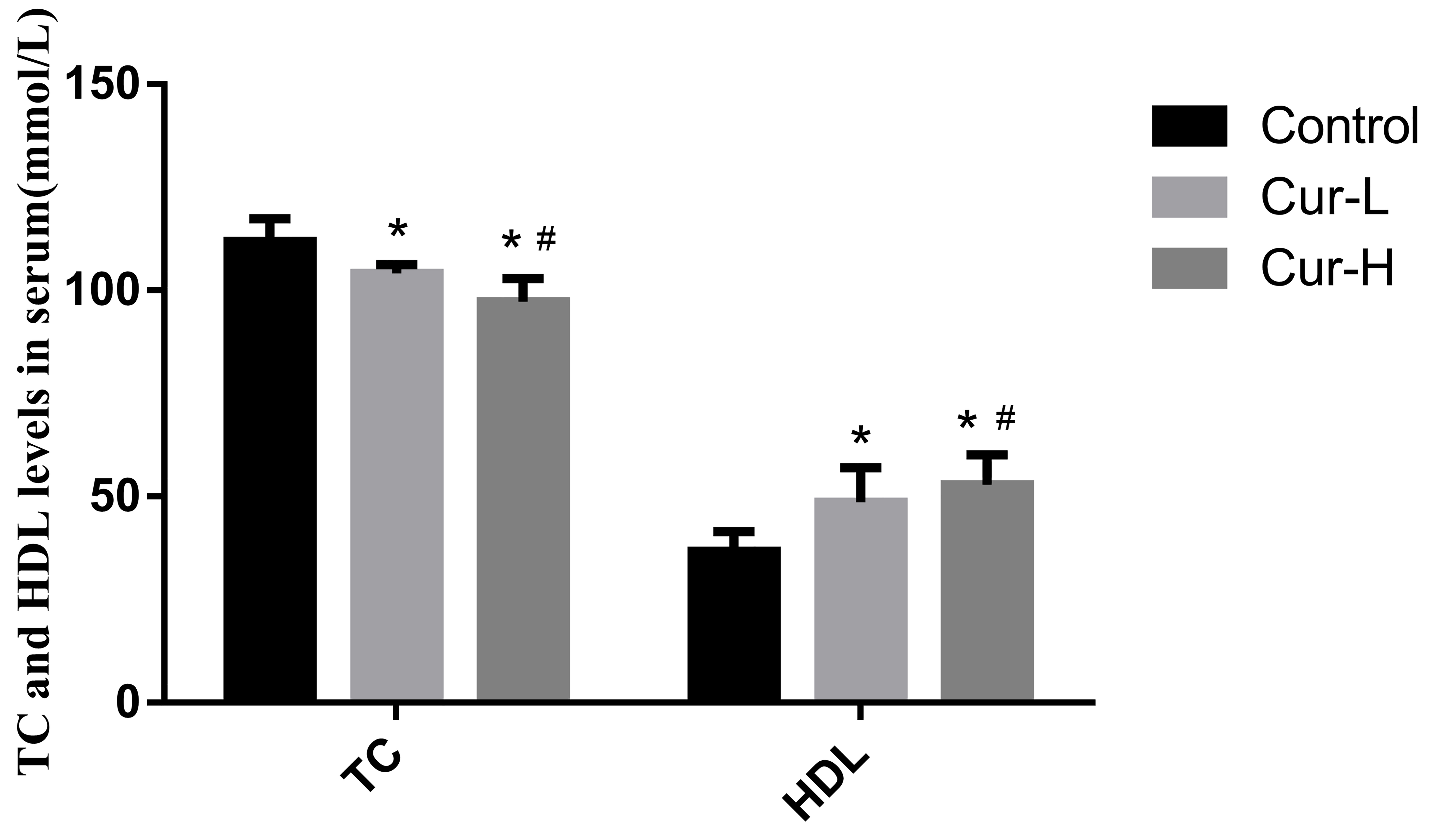 Fig. 3.
Fig. 3.Curcumin reduced the level of TC and increased HDL in the serum
of APP/PS1 mice (#p
Western blotting and immunocytochemistry were used to detect the ABCA1
expression and apoA1 in each group. Low-dose curcumin treatment enhanced the
expression of ABCA1 and apoA1 in the hippocampus of APP/PS1 double transgenic
mice (both p
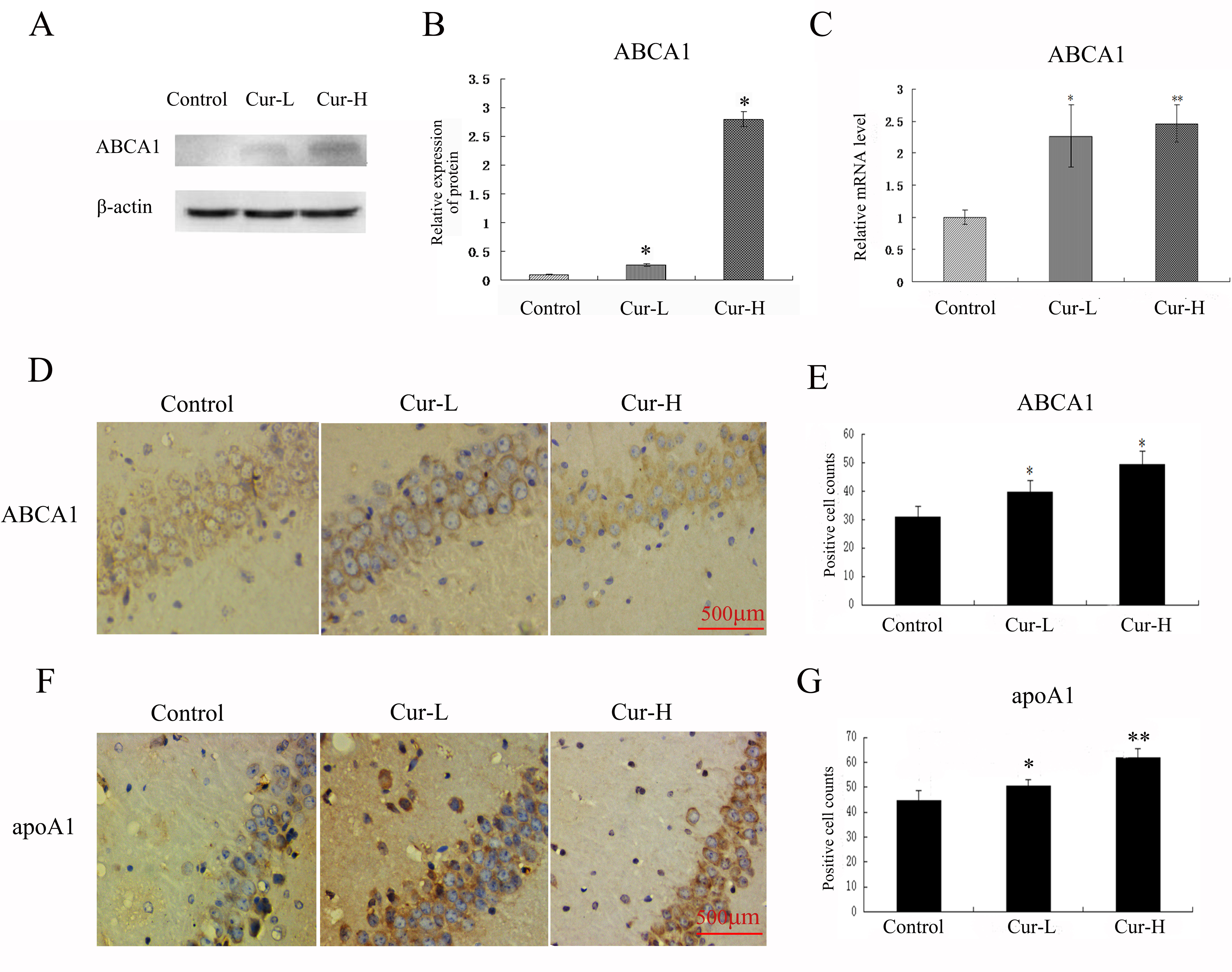 Fig. 4.
Fig. 4.Curcumin promoted the ABCA1 expression and apoA1 in the
hippocampus of APP/PS1 mice. (A–C) Western blot and RT-PCR analysis to ABCA1 of
each group. (D–G) Immunocytochemistry analysis of ABCA1 and apoA1 in each group
(Scale bar = 500
Western blotting, qRT-PCR and immunocytochemistry were used to evaluate the
expression of RXR-
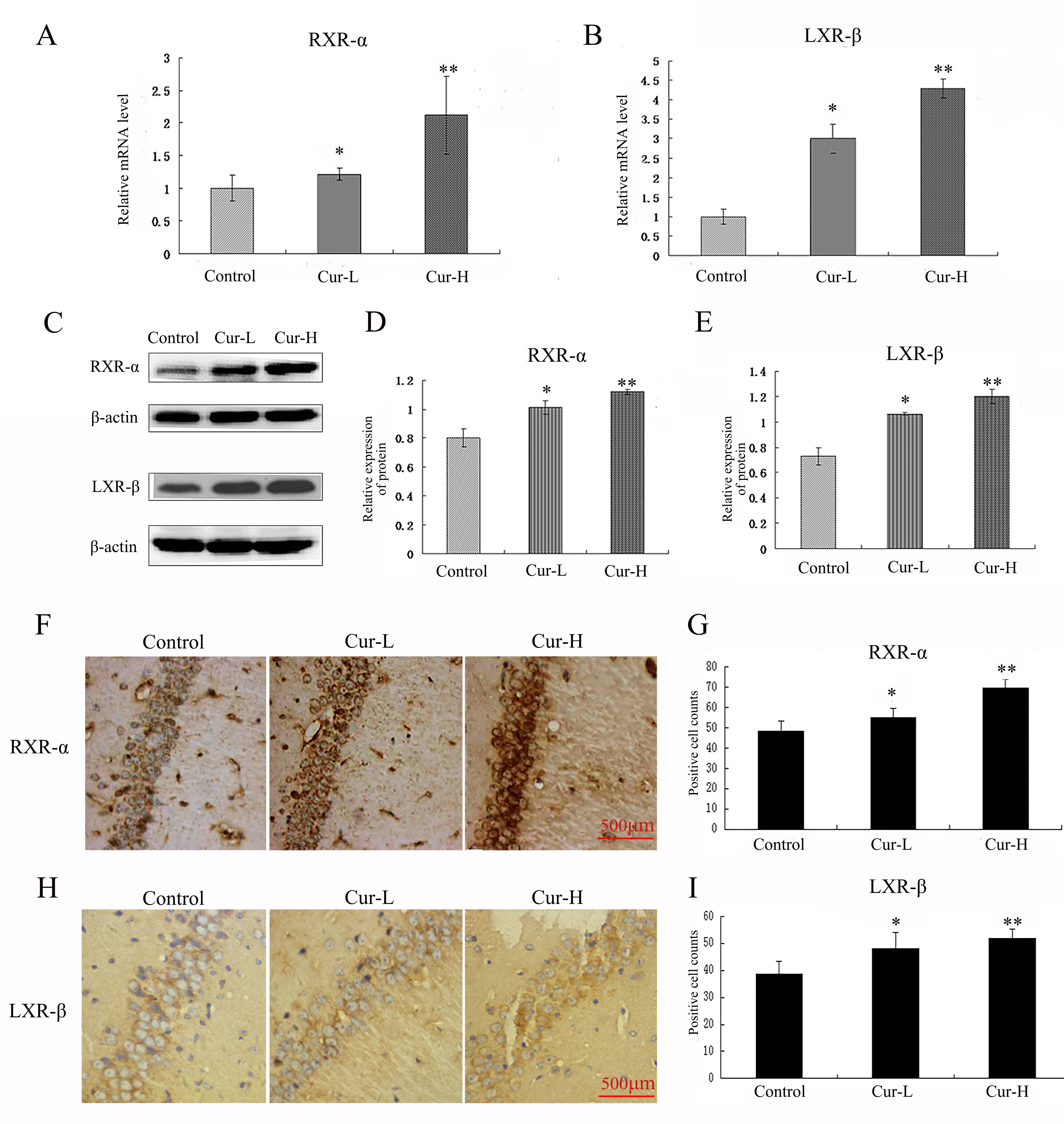 Fig. 5.
Fig. 5.Curcumin promoted the expression of RXR-
In neuroscience, it is assumed that the critical pathological change in AD is
the deposition of A
To further explore the protective effects of Curcumin against AD, the MWM test
suggested that curcumin improved cognitive function in the hippocampus of APP/PS1
mice. Curcumin also reduced A
Recent work suggests that AD is associated with cholesterol metabolism disorder. Cholesterol is an essential part of cell membranes and myelinated axons and plays an essential role in keeping the integrity and functionality of neurons [2]. However, the brain cannot obtain cholesterol from the blood due to the blood-brain barrier [4]. Synthesis by astrocytes and neurons is the primary source of cholesterol in brain tissues [20]. Epidemiological studies have found that patients with hypercholesterolemia were more likely to suffer from AD and that taking cholesterol-lowering drugs could reduce the incidence of AD [21, 22]. Transgenic mice with hyper cholesterol and rabbits on a high-cholesterol diet both showed cholesterol metabolism disorder that could promote amyloid deposition and lead to AD and cognitive dysfunction [23, 24]. The total cholesterol (TC) and low-density lipoprotein (LDL) levels of AD patients were higher than those of ordinary people [25]. These studies suggested an association between AD and cholesterol metabolism disorder. In the present research, curcumin reduced the TC level in APP/PS1 mice serum, with high-dose curcumin being more effective than low-dose curcumin. This finding is similar to Shin et al. [26], who found that curcumin significantly lowered the cholesterol level.
The ABCA1 transmembrane system is a one-way cholesterol transporter that
mediates outward transport after conjugating free cholesterol and phospholipid.
ABCA1 is a member of the ATP-binding cassette family [27]. Structurally, it has
two transmembrane segments and two nucleotide-binding domains. The
nucleotide-binding site can bind ATP to provide sufficient energy to support the
transmembrane transport of cholesterol. This way, ABCA1 substrates such as free
cholesterol and phospholipid can be transferred to the cell exterior [28]. ABCA1
is mainly regulated by LXR and RXR. The former is a nuclear receptor for oxidized
cholesterol, a member of the nuclear receptor superfamily, and a store of
cholesterol content in many kinds of cells. LXR has two subtypes: LXR-
Jiang et al. [33] found that amyloid deposition in the brain of aging
AD model mice (Tg2576) was dramatically decreased following treatment with
GW3965, an agonist of LXR. At the same time, the memory capacity of these
transgenic mice showed improvement. Fitz et al. [34] confirmed that
treatment with LXR agonist could reduce amyloid deposition in the brains of
transgenic mice fed a high-fat diet, as well as improve their cognitive function.
Fitz et al. [34] also reported that LXR agonists could promote the
activation of LXR-
We observed increased expression of RXR-
Following its transfer by ABCA1 to the extracellular fluid, cholesterol can
combine with ApoA1 bound to the cell surface to form HDL. This carries
cholesterol to the liver and reduces the cholesterol level due to the production
of bile [34]. The generally accepted view is that increased HDL levels and its
source protein apoA1 can lessen the probability of cardiovascular disease. Mature
HDL is the smallest spherical particle amongst plasma lipoproteins and can
scavenge intravascular lipid. Low HDL levels can lead to aberrant cholesterol
metabolism and lipid deposition [35]. Recent clinical studies have shown that HDL
and apoA1 levels in the plasma of AD patients are notably reduced [36]. Lewis
et al. [37] previously reported the HDL level in APP/PS1/apoA1
transgenic mice was double that observed in APP/PS1 transgenic mice. These
workers used the water maze test to confirm that APP/PS1/apoA1 transgenic mice
did not appear to have age-related learning and memory deficits, in direct
contrast to the results for APP/PS1 transgenic mice. Lewis et al. [37]
further suggested that plasma HDL and apoA1 levels were closely related to the
cognitive function of AD patients. Our results also indicate that HDL has a
protective effect against AD, since the HDL level increased as the level of
A
Our results show that curcumin can improve lipid metabolic disorder and cognitive dysfunction by promoting the ABCA1 transmembrane transport system (Fig. 6). Furthermore, curcumin may become an ideal medication to prevent and treat AD due to its multitarget activity as a natural agonist in the ABCA1 transmembrane transport system. One outstanding question that warrants further study is whether the ABCA1 transmembrane transport system is the most important mechanism by which curcumin can ameliorate AD symptoms. Therefore, we will continue to explore the impacts of curcumin on the ABCA1 transmembrane transport system and lipid metabolism in vivo, and it offers new ideas for the treatment of AD.
 Fig. 6.
Fig. 6.Schematic diagram about the curcumin’s possible mechanisms.
Curcumin could promote the expression of LXR-
Though there is essential evidence that curcumin may act on multiple pathways identified in AD’s pathogenesis, its low bioavailability and low blood-brain barrier (BBB) penetration limit the role of Curcumin in AD [38], studies exploring nanoparticle technology found that liposomal curcumin, polymeric nano curcumin and poly-lactic-co-glycolic acid co-polymer-curcumin could increase oral bioavailability or cross the BBB [39, 40]. Therefore, improving the bioavailability, BBB penetration and maintaining the half-life of curcumin is also a primary future goal.
AD, Alzheimer’s disease; ABCA1, ATP-binding cassette transporter A1; A
YL, FLZ, XZ and MYT designed the experiments; MYT, FLZ, ZPT, XZ, YW and CW performed the experiments; FLZ, MYT, ZPT and YYW helped in data analysis and paper writing. All authors have read and approved the manuscript.
Animals were raised and handled at the Chongqing University Cancer Hospital. All animal care protocols and experiments were reviewed and approved by the Ethics Committee of Chongqing University Cancer Hospital (CZLS2021244-A). The consent for publication was obtained from all authors.
We thank two anonymous reviewers for their excellent criticism of the article.
This work was supported by the National Natural Science Foundation of China (NSFC: 81671261, 81801389) and the Natural Science Foundation of Chongqing (No. cstc2017jcyjAX0050).
The authors declare no conflict of interest.
