Academic Editor: Peter A. McCullough
Trimethylamine N-oxide (TMAO) is reported to accelerate atherosclerosis and the
development of adverse cardiac outcomes. Relationship between coronary
atherosclerotic burden and TMAO has been examined in stable coronary artery
disease and ST-segment elevation myocardial infarction, but not in non-ST-segment
elevation myocardial infarction (NSTEMI). We examined the association between
TMAO and coronary atherosclerotic burden in NSTEMI. In this prospective cohort
study, two groups including NSTEMI (n = 73) and age-sex matched Healthy (n = 35)
individuals were enrolled between 2019 and 2020. Coronary atherosclerotic burden
was stratified based on the number of diseased coronary vessels and clinical risk
scores including SYNTAX and GENSINI. Fasting plasma TMAO was measured by isotope
dilution high-performance liquid chromatography. The median plasma TMAO
levels were significantly higher in the NSTEMI group than in the Healthy group,
respectively (0.59
Cardiovascular diseases (CVDs) remain a leading cause of death worldwide and their prevalence is increasing in the general population [1, 2]. The non-ST-segment elevation myocardial infarction (NSTEMI) is a frequent type of CVDs undergoing coronary intervention and reported with two-fold higher long-term adverse outcomes than patients with ST-segment elevation myocardial infarction (STEMI) [3, 4]. Nevertheless, sufficient consideration attributed to the reduction of the traditional risk factors including hyperlipidemia, smoking, hypertension, diabetes mellitus, and treatment with novel pharmacotherapies only decreased 30% of adverse outcomes related with CVDs [5, 6, 7]. Hence, identification of novel pathogenic risk factor related to CVDs has important public health significance for disease prevention and early stratification [8].
Recently, trimethylamine N-oxide (TMAO) metabolite gained widespread attention and purposed to play a potential role in adverse outcomes and pathogenesis for CVDs [9, 10, 11, 12, 13]. A significant association of the TMAO with the atherosclerotic burden in coronary vessels has been reported in STEMI and stable coronary artery disease (CAD) [14, 15]. However, the relationship between high coronary atherosclerotic burden and TMAO is yet to be explored in NSTEMI patients. In present study, we examined the relationship of the plasma TMAO levels with metrics that reflect coronary atherosclerotic burden, including the number of diseased coronary vessels and clinical risk scores including the SYNTAX and GENSINI scores in newly diagnosed NSTEMI patients.
This prospective cohort is a registered clinical study (ChiCTR1900022366) and received Ethics approval (YN201901) at the Fuwai Hospital Chinese Academy of Medical Sciences Shenzhen. All procedures were in accordance with the Declaration of Helsinki and each participant provided informed consent.
The main study protocol has been published in detail previously [16]. In brief,
two groups of individuals were prospectively enrolled including NSTEMI group (n =
73) and age-sex matched Healthy group (n = 35) between 2019 and 2020. The NSTEMI
group included patients with newly diagnosed NSTEMI, between 18 to 75 years old,
underwent coronary angiography (CAG) within 24 hours, with significant lesions
(
NSTEMI diagnosis was confirmed accordingly to European Society of Cardiology
guidelines including the presence of specific symptoms, changes in the
electrocardiogram (ECG) and myocardial biomarkers [1, 3, 4]. The symptoms
were specific angina related symptoms (chest discomfort or dyspnea) lasting more
than 30 minutes without accompanying persistent ST-segment elevation; changes in
ECG presented with ST-segment depression
The following baseline information including demographic, 12-lead ECG, physical examination, detailed present and past medical history, and blood samples were obtained in all individuals.
In the NSTEMI group, standard Fuwai hospital protocol was used to perform the
diagnostic CAG within 24 hours following admission via the redial approach. A
series of diagnostic angiogram projections were obtained including 4 views for
the left anterior descending (LAD) and left circumflex coronary (Cx), and 2 views
for the right coronary artery. All patients received dual antiplatelet therapy
[300 mg of aspirin (follow by 75 to 100 mg daily) and 600 mg of clopidogrel
(follow by 75 mg daily for
The coronary atherosclerotic burden was calculated using SYNTAX and GENSINI
scores by two expert cardiologists separately who were independent from this
study. The SYNTAX score was calculated by (www.syntaxscore.com). The GENSINI
score was obtained accordingly 1 point (1% to 25% stenosis), 2 points (26% to
50% stenosis), 4 points (51% to 75% stenosis), 8 points (76% to 90%
stenosis), 16 points (91% to 99% stenosis), and 32 points for a complete
occlusion [17]. These points were further multiplied according to the importance
of the coronary artery as 2.5 for proximal LAD artery and proximal Cx, 1.5 for
mid-LAD stenosis, and 1 for distal LAD, mid/distal Cx, and right coronary artery
stenosis [17]. Additionally, SYNTAX and GENSINI scores were further categorized
into intermediate-high risk (score
The blood samples were obtained from the peripheral veins with the subjects in
the fasting state on the morning after the day of admission, or prior any
procedure. Additionally, an extra EDTA sample was also collected at the same time
and immediately centrifuged at 2500 g at room temperature for 10 minutes and
obtained plasma sample was incubated at -80
The continuous and categorical data were displayed as mean
In this prospective cohort, the mean age (60.1
The baseline characteristics of the NSTEMI group stratified by MVD, SYNTAX and
GENSINI scores are shown in Table 1. The fifty-two patients (71.2%) had MVD and
twenty-one patients (28.7%) had SVD. The median SYNTAX score 23 (IQR: 11.5-29)
and median GENSINI score 38 (IQR: 20-64) were noted. Comparable baseline
characteristics were seen between MVD versus SVD, and intermediate-high risk
(score
The median plasma TMAO level (0.59
 Fig. 1.
Fig. 1.TMAO levels in NSTEMI and Healthy groups. TMAO, trimethylamine N-oxide.
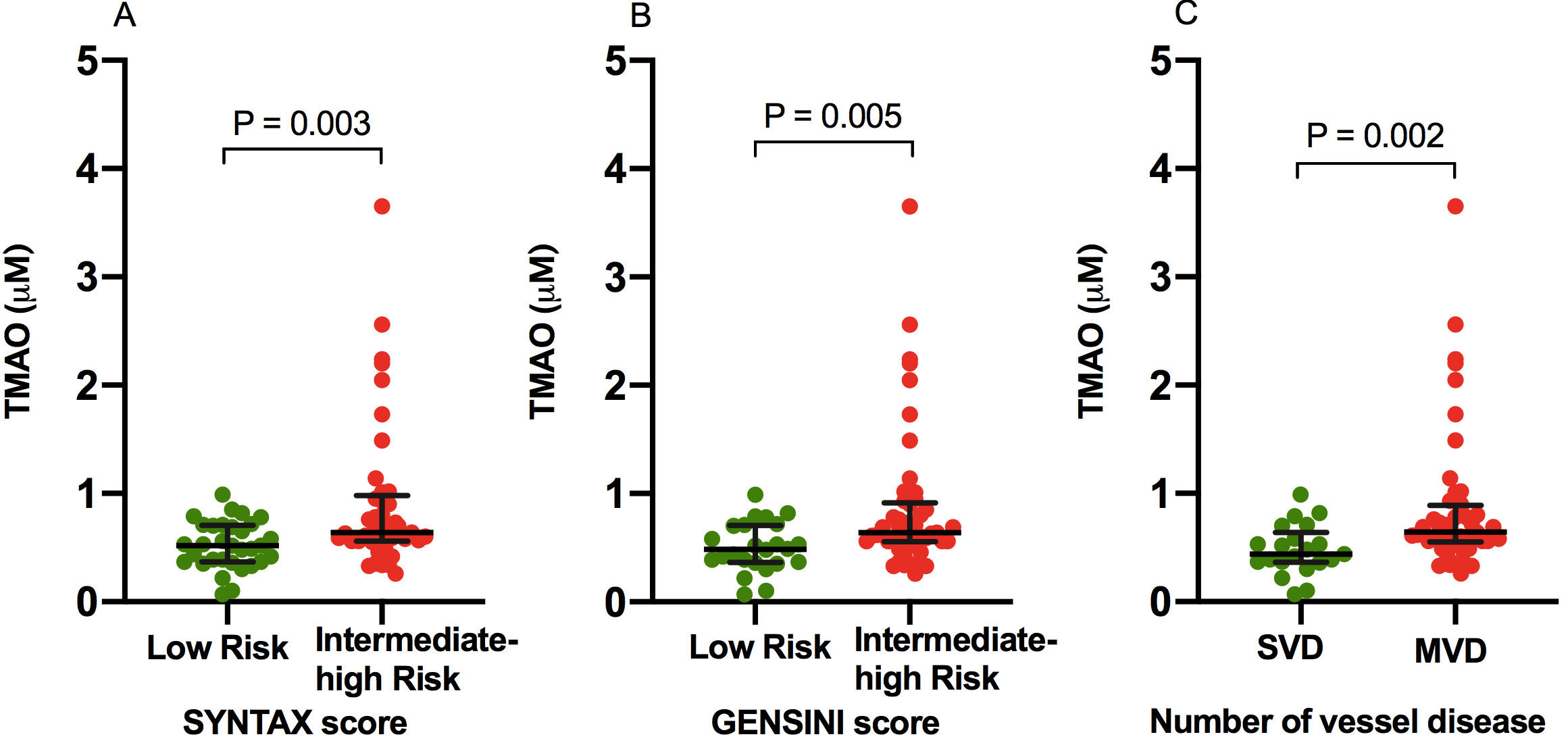 Fig. 2.
Fig. 2.Comparison of TMAO levels in Low versus Intermediate-High Atherosclerotic Burden. TMAO levels in low risk versus intermediate-high risk SYNTAX (A) and GENSINI (B) scores, and number of vessel disease (C). MVD, multivessel disease; SVD, single vessel disease; TMAO, trimethylamine N-oxide.
| Variables | Total (n = 73) | SYNTAX Score | GENSINI Score | MVD | ||||||
| P value | P value | NO (n = 21) | YES (n = 52) | P value | ||||||
| Age (Years) | 60.1 |
58.2 |
61.6 |
0.237 | 59 |
60.6 |
0.598 | 58.7 |
60.7 |
0.535 |
| Men | 61 (69.3%) | 27 (84.4%) | 34 (83.9%) | 0.564 | 20 (83.3%) | 41 (83.7%) | 0.607 | 17 (81%) | 44 (84.6%) | 0.473 |
| BMI (kg/m |
25 |
25.6 |
24.5 |
0.159 | 25.5 |
24.8 |
0.370 | 25.6 |
24.8 |
0.364 |
| SBP | 136.3 |
134.6 |
137.6 |
0.517 | 135.3 |
136.8 |
0.759 | 133.8 |
137.3 |
0.508 |
| DBP | 83.4 |
83.9 |
83 |
0.785 | 83.5 |
83.3 |
0.947 | 83.4 |
83.4 |
0.997 |
| Heart rate (bpm) | 71 (65-71) | 70 (65.2-76) | 72 (65-79) | 0.656 | 69 (64.2-75.7) | 73 (65-77) | 0.301 | 69 (63.5-74.5) | 73.5 (65-79.5) | 0.137 |
| NSTEMI Symptoms (days) | 3 (1-7) | 4 (1-7) | 2 (1-9.5) | 0.391 | 4 (2-6) | 2 (1-9.5) | 0.479 | 4 (1.5-6.5) | 2 (1-8.7) | 0.592 |
| Hypertension | 37 (50.7%) | 14 (43.8%) | 23 (56.1%) | 0.209 | 9 (37.5%) | 28 (57.1%) | 0.092 | 7 (33.3%) | 30 (57.7%) | 0.051 |
| Diabetes mellitus | 20 (27.4%) | 7 (22%) | 13 (31.7%) | 0.253 | 5 (20.8%) | 15 (30.6%) | 0.278 | 4 (19%) | 15 (30.8%) | 0.237 |
| Previous IS/TIA | 2 (2.7%) | 0 | 2 (4.9%) | 0.312 | 0 | 3 (4.1%) | 0.447 | 0 | 2 (3.8%) | 0.505 |
| Hyperlipidemia | 34 (46.6%) | 13 (40.6%) | 21 (51.2%) | 0.254 | 9 (37.5%) | 25 (51%) | 0.201 | 8 (38.1%) | 26 (50%) | 0.254 |
| Smoker | 24 (32.9%) | 14 (43.8%) | 10 (24.4%) | 0.067 | 10 (41.7%) | 14 (28.6%) | 0.196 | 10 (47.7%) | 14 (26.9%) | 0.078 |
| LVEF (%) | 60 (56.5-60) | 50 (59-60) | 60 (55-60) | 0.895 | 60 (60-60) | 60 (55-60) | 0.581 | 60 (59-60) | 60 (55-60) | 0.761 |
| eGFR (mL/min/1.73 m |
85.3 |
86.4 |
84.3 |
0.661 | 85.2 |
85.3 |
0.992 | 81.6 |
86.7 |
0.332 |
| TMAO (mM) | 0.59 (0.43-0.78) | 0.52 (0.37-0.70) | 0.64 (0.56-0.98) | 0.003 | 0.48 (0.36-0.70) | 0.64 (0.55-0.91) | 0.005 | 0.44 (0.36-0.64) | 0.64 (0.55-0.88) | 0.002 |
| hs-CRP (mg/L) | 2.8 (0.9-9.8) | 2.5 (0.9-9) | 3.3 (0.8-10.7) | 0.839 | 2.9 (1-8.4) | 2.7 (0.8-10) | 0.821 | 2.4 (0.9-6.2) | 3.3 (0.9-10.1) | 0.728 |
| D-dimer (mg/L) | 0.3 (0.2-0.5) | 0.3 (0.2-0.5) | 0.3 (0.2-0.49) | 0.281 | 0.3 (0.2-0.49) | 0.3 (0.2-0.5) | 0.198 | 0.2 (0.2-0.5) | 0.3 (0.2-0.5) | 0.675 |
| hs-TnI (ng/mL) | 0.8 (0.1-4.5) | 1.4 (0.2-4.3) | 0.3 (0.07-4.6) | 0.266 | 1.3 (0.3-2.9) | 0.3 (0.07-5.8) | 0.336 | 0.8 (0.3-2.9) | 0.3 (0.07-6.5) | 0.457 |
| hs-TnT (ng/mL) | 0.1 (0.04-0.5) | 0.2 (0.05-0.5) | 0.1 (0.038-0.6) | 0.347 | 0.2 (0.06-0.5) | 0.1 (0.03-0.6) | 0.354 | 0.1 (0.06-0.6) | 0.1 (0.03-0.58) | 0.330 |
| NT-proBNP (pg/mL) | 333 (128-623) | 197 (105-495) | 425 (181-673) | 0.053 | 232 (81-621) | 360 (174-624) | 0.139 | 256 (68-589) | 336 (144-629) | 0.167 |
| TC (mmol/L) | 4.4 (3.7-5.2) | 4.4 (3.7-5.2) | 4.4 (3.7-5.3) | 0.947 | 4.5 (3.9-5.3) | 4.1 (3.6-5.2) | 0.354 | 4.2 (3.8-5) | 4.4 (3.6-5.3) | 0.622 |
| TG (mmol/L) | 1.7 (1.1-2.3) | 1.7 (1.1-2.5) | 1.6 (1-2.2) | 0.339 | 1.6 (1.1-2.28) | 1.7 (1.1-2.48) | 0.729 | 1.6 (1.1-2.7) | 1.7 (1.1-2.3) | 0.992 |
| LDL (mmol/L) | 2.8 (2.4-3.7) | 2.9 (2.5-3.8) | 2.4 (2.4-3.6) | 0.420 | 3 (2.6-4) | 2.8 (2.4-3.6) | 0.243 | 2.8 (2.4-3.6) | 2.9 (2.4-3.7) | 0.807 |
| HDL (mmol/L) | 1 (0.9-1.2) | 1 (0.8-1.1) | 1 (0.9-1.2) | 0.158 | 1 (0.9-1.2) | 1 (0.8-1.2) | 0.851 | 1 (0.9-1.2) | 1 (0.8-1.1) | 0.961 |
| Continues data are expressed as means | ||||||||||
Multivariate logistic regression adjusting analysis found that plasma TMAO level
was independently associated with MVD (odds ratio [OR]: 5.94, P =
0.005), and intermediate-high risk (score
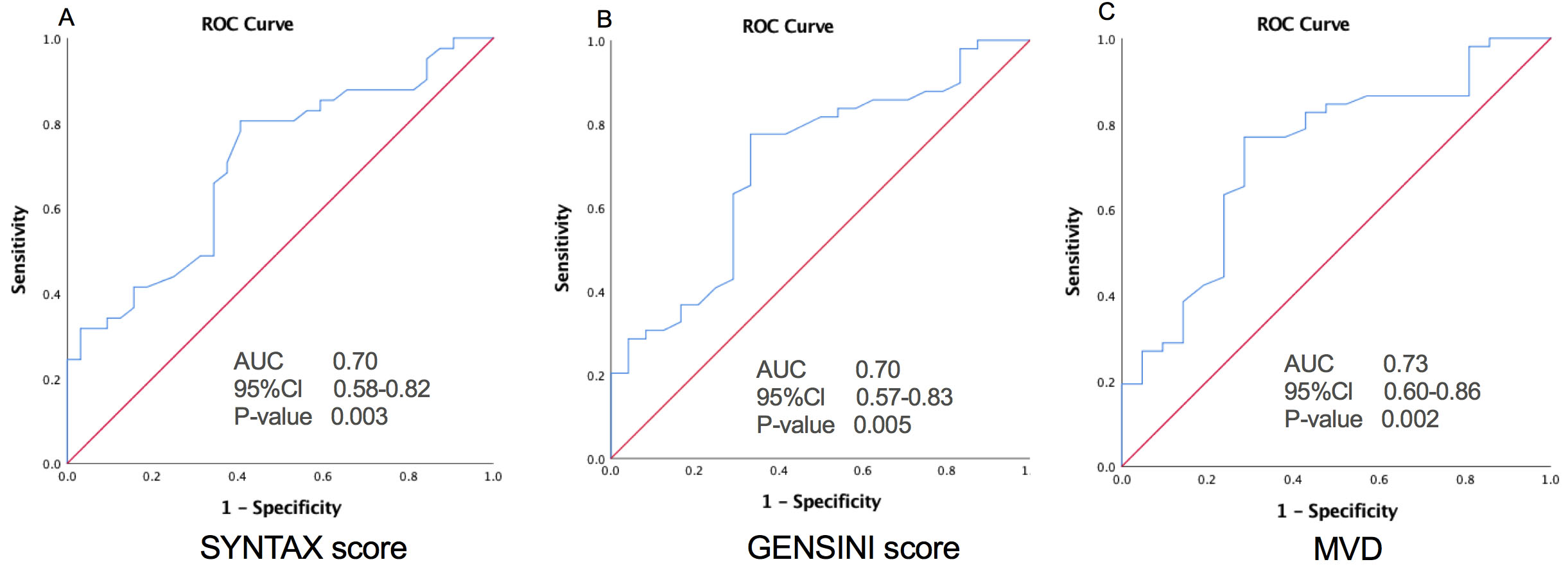 Fig. 3.
Fig. 3.Relationship of TMAO with High Atherosclerotic Burden. The ROC curves of TMAO for predicting a high atherosclerotic burden as intermediate-high risk SYNTAX (A) and GENSINI (B) scores, and MVD (C). AUC, area under the receiver-operating characteristic curve; Cl, confidence interval; MVD, multivessel disease.
| SYNTAX score | GENSINI score | MVD | |||||
| OR (95% Cl) | P value | OR (95% Cl) | P value | OR (95% Cl) | P value | ||
| TMAO (mM) | Unadjusted | 2.85 (0.66-5.04) | 0.011 | 3.03 (0.59-5.47) | 0.015 | 3.63 (0.883-6.38) | 0.010 |
| Adjusted* | 3.61 (0.74-6.47) | 0.013 | 4.60 (1.19-8.00) | 0.008 | 5.94 (1.79-10.10) | 0.005 | |
| *Adjusted for traditional risk factors including age, sex, diabetes,
hypertension, smoking, TG, LDL, BMI, hs-CRP, eGFR.
Cl, confidence interval; OR, odds ratio; and other abbreviations are listed in Table 1. | |||||||
The main finding of this study was a significant association between plasma TMAO
levels and high coronary atherosclerotic burden as stratified by number of
disease vessels and clinical risk scores including SYNTAX and GENSINI scores in
NSTEMI patients. Moreover, TMAO independently predicted the MVD, and
intermediate-high risk (score
Atherosclerosis is a significant clinical problem leading to ischemia in different parts of the vasculature [19, 20, 21]. In this occurs in the coronary vessels, then CAD with calcification [22], partial or total vessel occlusion [23, 24, 25], leading to gradual reduction or absent coronary blood flow [21, 26]. This can manifest as silent asymptomatic disease, angina pectoris [27, 28], NSTEMI or STEMI [29, 30, 31], with adverse consequences such as arrhythmias, heart failure, and death [32]. Therefore, there is an increasing interest to develop predictive risk models for accurate risk stratification, which may include clinical signs or symptoms, imaging results and biomarkers [33]. The TMAO is a gut metabolite, generated from dietary nutrients such as choline, L-carnitine, betaine, and phosphatidylcholine [9, 34, 35]. There is growing appreciation that TMAO has mechanistic links to proatherogenic effect via increased macrophage foam cell formation, activation of the inflammatory and platelet hyperactivity pathways, impaired cholesterol and bile acid transport [9, 10, 11, 12, 34, 35, 36, 37]. These all impaired factors are associated with high-risk CVDs including stroke, atherosclerosis, atrial fibrillation, heart failure, and chronic kidney disease [9, 10, 11, 12, 20, 34, 35, 36, 37, 38]. Based on experimental animal studies, TMAO accelerates atherosclerotic progression by inhibiting reverse cholesterol transport, enhancing platelet activity and thrombosis, and activating macrophages, while targeting production of TMAO inhibit pathogenesis of atherosclerotic [9, 10, 34, 35, 36, 37]. Recent clinical data demonstrated a close relationship of TMAO with markedly high short and long-term adverse outcomes related to CVDs even following adjustment for confounding risk variables [11, 12, 13, 38]. More recently, Yoriko et al. further appraised that long-term increases in TMAO were linked to a higher risk for CVDs and repeated evaluation of TMAO over 10 years increased the early identification of individuals with a greater CVD-related risk [39].
This study is the first, to the best of the authors’ knowledge, to evaluate the
relationship between TMAO and coronary atherosclerotic burden in NSTEMI patients.
Our study further contributes to earlier findings those suggested a significant
link between plasma TMAO and atherosclerotic burden in STEMI and stable CAD
patients [14, 15]. Sheng et al. showed a significant association between
plasma TMAO levels and high atherosclerotic burden as ascertained by the SYNTAX
score and MVD in STEMI [14]. Senthong et al. noted that strong
predictability of TMAO for high coronary atherosclerotic burden as evaluated via
SYNTAX score stable CAD [15]. Lately, Guo et al. also observed a
positive linked of TMAO level with of a high atherosclerotic burden particularly
severe coronary artery lesion (
The strength of our study that TMAO was evaluated in multiple parameters for
quantification and stratification of coronary atherosclerotic burden including
clinical risk scores such as SYNTAX and GENSINI, and MVD as compared to prior
studies evaluated TMAO in only SYNTAX score and/or MVD [14, 15]. A high
SYNTAX and GENSINI scores, and MVD are associated with poor prognosis and
considered as clinical markers of coronary atherosclerotic burden [3, 17, 41].
Notably, the median SYNTAX score 23 (IQR: 11.5-29) in NSTEMI patients recruited in
the present cohort were seemingly higher than STEMI patients described by Sheng
et al. 18 (IQR: 11-23.5) and stable CAD patients by Senthong et
al. 11 (IQR: 4-18.5), which might be partially attributed to higher proportion
of patients (56.1%) in intermediate-high risk SYNTAX score category in the
present cohort compared to Sheng et al. (27.8%) and Senthong et
al. (18.1%) [14, 15]. Moreover, our study included NSTEMI patients with
significant coronary lesion (
Recently, there is accumulating evidence to suggest TMAO is a clinically relevant target or marker for risk stratification and short or long-term prognostic outcome related in CVDs [11, 12, 14, 15]. Moreover, inhibition of TMAO has been identified as a novel therapeutic approach for the prevention of atherosclerosis progression compared with existing traditional treatment [10, 11, 12, 37, 39, 43, 44, 45, 46]. In light of these promising findings, implication of TMAO may establish early diagnosis, risk stratification, optimal treatment, and preventive strategies for CVDs [10, 11, 12, 37, 39, 43, 44, 45, 46].
Firstly, we were unable to assess the nutritional and diet status before enrollment for each individual that may bias results as TMAO may be influenced by nutritional status as previously reported [43, 44, 45, 46]. Secondly, we could not exclude selection bias as the present cohort is a single-center study with a comparatively small number of NSTEMI patients, particularly in all sub-categorized groups. Thirdly, the COVID-19 pandemic unable us to continue and complete our original SZ-NSTEMI trial including enrollment of NSTEMI patients about two hundred participants for the evaluation of TMAO in regards to short and long-term prognostic outcomes after primary percutaneous coronary intervention as previously described [16]. Therefore, we recommended a large prospective randomized control trial to evaluate the association between TMAO and coronary atherosclerotic burden as well as a prognostic biomarker for adverse cardiac outcomes in NSTEMI patients undergoing coronary intervention.
In conclusion, our study demonstrated that plasma TMAO levels were significantly associated with a high coronary atherosclerotic burden as quantified by the number of diseased vessels, and clinical risk scores including SYNTAX and GENSINI scores in patients with NSTEMI. We encouraged and warranted future large, randomized trial to evaluate our observed results in NSTEMI patients for the clinical endpoints.
CAD, coronary artery disease; CAG, coronary angiography; CVDs, cardiovascular diseases; ECG, electrogram; MVD, multiple vessels disease; NSTMEI, non-ST-segment myocardial infarction; STEMI, ST-segment myocardial infarction; SVD, single vessel disease; SYNTAX, SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery; TMAO, trimethylamine N-oxide.
Lili W, Junlei C, and Bin Waleed K were involved to research design, protocol, submission to ethical committee, and trial registration. Bin Waleed K, Pengnong C, Tu H, Ding L, Yangkong L, Houqing Z, Qiying C, and Aimei S contributed to literature review, patients selection accordingly to inclusion and exclusion criteria, informed consent, and data collection and interpretation of data. Yunlong X, Shulin W, Tse G, Xintao L, and Afrasyab A underwent statistical analysis and manuscript writing. All authors are involved in final draft of the manuscript, read and approved the final manuscript.
The study was conducted in accordance with the Declaration of Helsinki and the Ethics Committee of Fuwai Hospital Chinese Academy of Medical Sciences Shenzhen (Ethics approval number: YN201901) and registered clinical study (ChiCTR1900022366) as SZ-NSTEMI. The study can be identified http://www.chictr.org.cn/showprojen.aspx?proj=37821. Written informed consent was obtained from all participants.
We thanked all participated individuals in this study.
This study was funded by the Science Technology and Innovation Commission of Shenzhen Municipality (ZDSYS20190902093409851), Guangdong Innovation Platform of Translational Research for Cerebrovascular Diseases, and National Natural Science Foundation of China (81971634).
The authors declare no conflicts of interest.
