Academic Editor: Gary David Lopaschuk
Older age is known as a negative prognostic parameter in acute myocardial
infarction (AMI) patients. In this study, we aimed to explore age-associated
differences in treatment protocols, in-hospital and 1-year mortality. This cohort
observational study included 277 consecutive AMI patients, separated into 2
groups according to whether their age was
Elderly people are a fast-rising part of the population. They represent a rapidly increasing amount of patients with acute coronary syndromes (ACS), including myocardial infarction with ST-elevation (STEMI) and without ST-elevation (NSTEMI). Advanced age is a potent predictor of bad prognosis. The majority of AMI trials have enrolled an unimportant number of elderly subjects, and this explains why incomplete data are available on the management and prognosis of this increasing subset of the ACS population.
Standard therapies are not always applied in the elderly, as evidence of benefit is lacking and the risk of serious side effects is high for this age group [1, 2, 3]. These facts can be also explained by some specific clinical characteristics of the elderly at presentation: the symptoms of the ACS are less specific, the electrocardiographic patterns are more often atypical and the comorbidities may lead to a confounding clinical picture. All these facts may lead to diagnostic incertitude and delayed or conservative therapeutic strategies.
This situation can be found in Romania to, though aggravated by the small number
of catheterization laboratories that are able to perform urgent coronary
revascularization (23, for a population of 20 million inhabitants). Our
retrospective study is the first one done in Romania addressing AMI patients aged
This is a cohort study. From 1st January to 31st December of 2019, all
successive patients that were hospitalized with AMI at Timisoara Institute of
Cardiovascular Disease were evaluated for enrolling in the study. In the absence
of contraindications, percutaneous coronary intervention (PCI) was performed in
the first 12 hours from the symptoms onset in all STEMI and in the high-risk
NSTEMI patients. As high-risk NSTEMI patients were stated those with at least one
of the following criteria: a GRACE-score
The initial evaluation was based on the analysis of the parameters resulting
from clinical presentation, cardiac troponin, and resting 12-lead ECG [4, 5]. The
diagnosis of AMI with persistent ST-segment elevation (STEMI) was stated in the
presence of at minimum 2 of the subsequent 3 parameters: (1) typical angina
lasting over 20 minutes; (2) ST-segment elevation
AMI without persistent ST-segment elevation (NSTEMI) was diagnosed in the
presence of an appropriate clinical situation (typical angina or angina
equivalent) with ST‑segment depression or prominent T‑wave inversion, without
ST‑segment elevation and/or positive biomarkers of necrosis (e.g., troponin I
The PCI and the adjunctive pharmacological medication was performed according to the ESC guidelines. The patients were administered standard loading doses of 300–600 mg clopidogrel, 300–500 mg aspirin and 5000 IU unfractionated heparin before the PCI. Glycoprotein IIb/IIIa inhibitors were given when the operator considered it necessary. If a coronary stent was implanted, clopidogrel was prescribed for 12 months, associated with aspirin.
The inclusion criteria were the confirmed diagnosis of AMI hospitalized within the first 12 hours of the symptoms onset and the absence of exclusion criteria.
Exclusion criteria were: PCI‑ or CABG- related AMI [7], and the association of diseases worsening the long‑term prognosis such as severe primary cardiomyopathy, severe valvular diseases congenital heart diseases, kidney dysfunction, liver cirrhosis, a malignant tumor, and severe infection.
Baseline data were taken from hospital records and comprised gender, age, Killip functional class on admission, medical history, 12 leads resting electrocardiogram, laboratory data, echocardiographic data, and the results of the coronary angiography.
Medical history integrated data regarding obesity, smoking, old myocardial infarction, hypertension, peripheral vascular disease, chronic obstructive pulmonary disease, diabetes, chronic kidney disease, stroke. The laboratory data determined at admission were creatine kinase-MB isoenzyme and cardiac troponin levels, blood cell count, serum hemoglobin, serum glucose, liver enzymes, serum creatinine, serum electrolytes, and lipogram.
Echocardiographic evaluation was done within the first 24 hours of hospitalization, using a VIVID S5 echocardiograph. Mono- and two dimensional echocardiography, as well as pulsed and continuous Doppler imaging, were performed in all patients. Left ventricular ejection fraction (LVEF) was calculated using the Simpson method, evaluating the end-diastolic volume and left ventricular end-systolic volume [8].
Medical treatment reports were accomplished at discharge and 1 year follow up.
The cause of death was determined from hospital records, or by asking the patient‘s physician for those who died at home.
All causes of readmissions were noted during the 1-year follow-up period. The causes of readmissions were determined by utilizing the hospital records.
The primary endpoint was in-hospital mortality, defined as the death of any cause during the hospitalization for AMI.
Cardiac deaths were considered those due to AMI, heart failure, acute pulmonary edema, cardiogenic shock, ventricular fibrillation, or cardiac rupture.
Noncardiac deaths were considered those having an extra-cardiac cause, such as stroke, acute renal failure, or sepsis.
The secondary endpoints were the 1-year death and readmission rates. 1-year mortality included both cardiac and non-cardiac deaths. 1-year readmissions had as possible causes recurrent myocardial infarction (RMI), stroke, stent thrombosis, and bleeding. RMI was defined according to the Academic Research Consortium (ARC) criteria [9]. Bleeding complications were defined according to the BARC and the TIMI bleeding classification [10, 11]. Stroke was stated as an irreversible neurological impairment, as confirmed by the neurologist, and based on sustaining information, such as brain images.
Data were collected and analyzed by means of the MedCalc Statistical Software
version 19.1.7 for Windows. Ostend, Belgium. Data are given as mean
Of the 297 patients hospitalized with AMI, 277 were enrolled in the study. The
mean age was 67.38
| Group I | Group II | P value | ||
| Age |
Age |
|||
| n = 063 | n = 214 | |||
| Mean age, years (Χ |
83.7 |
62 |
||
| Male sex (n, %) | 23 (36%) | 151 (70%) | ||
| Smokers (n, %) | 13 (20%) | 115 (54%) | ||
| Obesity (n, %) | 13 (20%) | 55 (25%) | 0.41 | |
| Diabetes mellitus ( n, %) | 77 (29%) | 64 (30%) | 0.87 | |
| Hypercholesterolemia ( n, %) | 48 (75%) | 149 (70%) | 0.43 | |
| COPD (n, %) | 19 (30%) | 53 (25%) | 0.42 | |
| Chronic kindey disease (n, %) | 19 (30%) | 27 (12%) | ||
| Systemic hypertension (n, %) | 58 (90%) | 168 (78%) | 0.03 | |
| Peripheral artery disease (n, %) | 4 (6%) | 12 (6%) | 1 | |
| History of stroke (n, %) | 20 (31%) | 27 (13%) | ||
| Old myocardial infarction (n, %) | 10 (14%) | 24 (11%) | 0.51 | |
| Previous PCI (n, %) | 5 (8%) | 15 (7%) | 0.78 | |
| Previous CABG (n, %) | 2 (3%) | 3 (1.4%) | 0.39 | |
| Known congestive heart failure | 22 (34%) | 28 (13%) | 0.0001 | |
| STEMI (n, %) | 54 (84%) | 196 (92%) | 0.05 | |
| High-risk NSTEMI (n, %) | 9 (13%) | 18 (8%) | 0.22 | |
| Killip class at admission | 2.6 |
2.2 |
||
| Heart rate at admission (Χ |
82.6 |
79.4 |
0.23 | |
| Systolic BP at admission (Χ |
124.3 |
130.7 |
0.08 | |
| Diastolic BP at admission (Χ |
75.2 |
77.1 |
0.38 | |
| Atrial Fibrillation at admission (n, %) | 22 (34%) | 30 (14%) | 0.07 | |
| - acute (n, %) | 12 (19%) | 17 (8%) | 0.29 | |
| - persistent (n, %) | 10 (15%) | 13 (6%) | 0.17 | |
| Recenl LBBB at admission (n, %) | 1 (1.5%) | 4 (1.7%) | 0.27 | |
| AV block at admission (n, %) | 5 (7.5%) | 12 (6%) | 0.26 | |
| - 2nd degree (n, %) | 1 (1.5%) | 4 (2%) | 1 | |
| - 3rd degree (n, %) | 4 (6%) | 8 (4%) | 0.19 | |
| Ventricular fibrillation at admission (n, %) | 6 (9%) | 17 (8%) | 0.56 | |
| LVEF at admission | 38 |
47 |
||
| 47 (73%) | 100 (47%) | |||
| 40–49% at admission | 9 (14%) | 62 (29%) | 0.01 | |
| 8 (13%) | 52 (24% | 0.06 | ||
| Scr (mg/dL, mean |
2.2 |
1.72 |
0.02 | |
| BNP (pg/mL, mean |
881.69 |
834.79 |
0.20 | |
| CK-MB ( |
50.01 |
51.34 |
0.52 | |
| Tpn‑I ( |
14.98 |
14.69 |
0.38 | |
| Note: Statistically significant values are written in bold (P Abbreviations: AMI, acute myocardial infarction; ACEI, Angiotensin‑converting enzyme inhibitor; ARB, Angiotensin receptor blocker; AV, atrio-ventricular; BNP, Brain natriuretic peptide; BP, blood pressure; CABG, Coronary artery bypass grafting; CCB, Calcium antagonists; CK‑MB, Creatine kinase‑MB; E/A - the ratio of peak velocity blood flow in early diastole to peak velocity flow in late diastole; LBBB, left ventricular blood pressure; LVEF, Left ventricular ejection fraction; LWWH, Low molecular weight heparin; NSTEMI, acute myocardial infarction without ST-segment elevation; PCI, Percutaneous coronary intervention; Scr, Serum creatinine; STEMI, acute myocardial infarction woth persistent ST-segment elevation; Tpn‑I, Troponin‑I. | ||||
Table 2 describes the findings of the coronary angiography performed within the
first 12 hours from the onset of symptoms and the therapeutical interventionsf.
No angiography could be done in 3 (4%) of group I patients and in 1 (0.5%)
patients of group II (P = 0.03), because of severe kindney failure.
Group I patients presented a significantly higher prevalence of 3-vessel coronary
disease (P = 0.01), and a significantly lower prevalence of myocardial
revascularization by PCI (48% vs. 78%, P
| Group I | Group I | P value | ||
| Age |
Age |
|||
| n = 63 | n = 214 | |||
| Door to baloon time (min) | 83 |
80 |
0.06 | |
| Radial approach n (%) | 19 (52%) | 102 (48%) | 0.57 | |
| Angiographic findings- number of diseased vessels | 61 (96%) | 213 (99.5%) | 0.03 | |
| One | 23 (36%) | 104 (49%) | 0.06 | |
| Two | 7 (11%) | 48 (22%) | 0.05 | |
| Three | 21 (32%) | 38 (18%) | 0.01 | |
| Left main trunk | 10 (16%) | 23 (11%) | 0.28 | |
| Interventional revascularization: | 32 (50%) | 174 (81%) | ||
| PCI | 31 (48%) | 167 (78%) | ||
| CABG | 1 (2%) | 7 (3%) | 0.67 | |
| Concomitant drug therapy | ||||
| Clopidogrel | 63 (100%) | 210 (98%) | 0.25 | |
| Aspirin | 62 (97%) | 210 (98%) | 0.63 | |
| Betablockers | 48 (76%) | 175 (82%) | 0.29 | |
| Statin | 60 (95%) | 206 (96%) | 0.72 | |
| ACEI/BRA | 46 (72%) | 156 (73%) | 0.87 | |
| CCB | 16 (25%) | 64 (30%) | 0.44 | |
| Diuretics | 42 (65%) | 54 (25%) | ||
| Oral anticoagulants | 22 (35%) | 30 (14%) | 0.0002 | |
| Note: Statistically significant values are written in bold (P Abbreviations: ACEI, Angiotensin‑converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, Angiotensin receptor blocker; AV, atrio-ventricular; BNP, Brain natriuretic peptide; BP, blood pressure; CABG, Coronary artery bypass grafting; CCB, Calcium antagonists; CK‑MB, Creatine kinase‑MB; E/A - the ratio of peak velocity blood flow in early diastole to peak velocity flow in late diastole; LBBB, left ventricular blood pressure; LVEF, Left ventricular ejection fraction; LWWH, Low molecular weight heparin; NSTEMI, acute myocardial infarction without ST-segment elevation;PCI, Percutaneous coronary intervention; Scr, Serum creatinine; STEMI, acute myocardial infarction with persistent ST-segment elevation; Tpn‑I, Troponin‑I. | ||||
The total all-cause mortality rate was 12.6% (n = 35). The number of deaths was
18 (28%) in the octogenarians, besides 17 (8%) in the non-octogenarians,
P
During the hospitalization for AMI, 26 patients (9.3%) died, 12 being from
group I (19%), and 14 from group II (6.5%), P
 Fig. 1.
Fig. 1.Kaplan-Meyer curves for in-hospital mortality in AMI patients.
Abbreviations: AMI, acute myocardial infarction.
| Group I | Group II | P value | ||
| Age |
Age |
|||
| n = 63 | n = 214 | |||
| Total mortality | 18 (28%) | 17 (8%) | ||
| In hospital mortality | 12 (19%) | 14 (6.5%) | ||
| Cardiac causes | 7 (11%) | 8 (3.7%) | 0.02 | |
| Ventricular fibrillation | 3 (5%) | 3 (1.4%) | 0.08 | |
| Electromechanical dissociation | 1 (1.5%) | 1 (0.4%) | 0.34 | |
| Cardiogenic shock | 2 (3%) | 3 (1.5%) | 0.43 | |
| Acute pulmonary edema | 1 (1.5%) | 1 (0.4%) | 0.34 | |
| Noncardiac causes | 5 (8%) | 6 (2.8%) | 0.04 | |
| Acute renal failure | 2 (1.1%) | 2 (0.9%) | 0.88 | |
| Bleeding | 1 (0.7%) | 1 (0.5%) | 0.85 | |
| Stroke | 1 (0.3%) | 1 (0.5%) | 0.63 | |
| Sepsis | 1 (0.3%) | 2 (0.9%) | ||
| Discharged patients | Group I | Group I | P value | |
| Age |
Age |
|||
| n = 51 | n = 200 | |||
| Medication at discharge | ||||
| Clopidogrel | 41 (80%) | 170 (85%) | 0.38 | |
| Aspirin | 44 (86%) | 168 (84%) | 0.72 | |
| Betablockers | 33 (64%) | 146 (73%) | 0.20 | |
| Statin | 42 (81%) | 172 (86%) | 0.37 | |
| ACEI/BRA | 37 (72%) | 156 (78%) | 0.36 | |
| Oral anticoagulants | 13 (26%) | 24 (12%) | 0.01 | |
| CCB | 20 (40%) | 62 (31%) | 0.22 | |
| Diuretics | 20 (40%) | 52 (26%) | 0.04 | |
| 1-year mortality | 6 (12%) | 3 (1.5%) | 0.0004 | |
| Causes: | ||||
| Recurrent myocardial infarction | 2 (4%) | 2 (1%) | 0.12 | |
| Congestive heart failure | 2 (4%) | 1 (0.5%) | 0.04 | |
| Stroke | 1 (2%) | - | 0.04 | |
| Bleeding | 1 (2%) | - | 0.04 | |
| Note: Statistically significant values are written in bold (P Abbreviations: ACEI, Angiotensin‑converting enzyme inhibitor; ARB, Angiotensin receptor blocker; CCB, Calcium antagonists. | ||||
In univariate analysis, the variables associated with in-hospital death were age
The multivariate logistic regression selected three parameters as independent
predictors for in-hospital mortality. These parameters were: an age
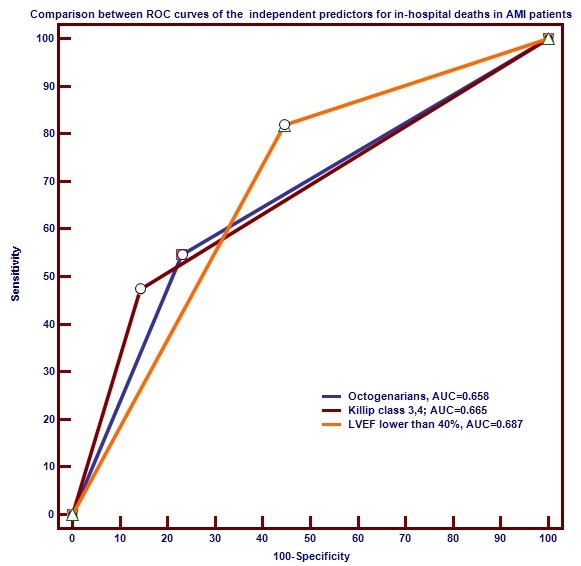 Fig. 2.
Fig. 2.Comparison between ROC curves of the independent predictors for
in-hospital death in AMI patients.
Abbreviations: AMI, acute myocardial infarction; AUC, area under the curve;
LVEF, left ventricular ejection fraction; ROC, receiver operating characteristic.
251 AMI patients were discharged alive (90%) and followed-up for 1 year. Group
I patients received at discharge more often oral anticoagulants (P =
0.01) and diuretics (P = 0.04). During the follow-up interval further 9
patients died, thus the 1-year mortality was 3.6%. 1-year mortality was notably
higher in group I patients (12% vs. 1.5%, P
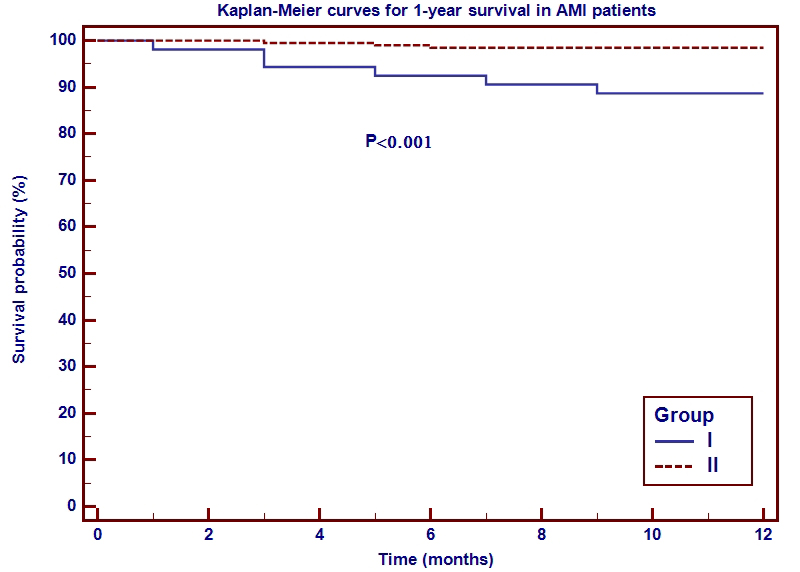 Fig. 3.
Fig. 3.Kaplan-Meyer curves for 1-year mortality in AMI patients.
Abbreviations: AMI, acute myocardial infarction.
The 1-year mortality risk was associated in univariate analysis with
triple-vessel disease (P = 0.002), Killip class
The multivariate logistic regression selected two parameters as independent
predictors for the 1-year mortality: the triple-vessel CAD (OR = 3.3, 95% CI:
1.47 to 7.75, P = 0.004) and an LVEF
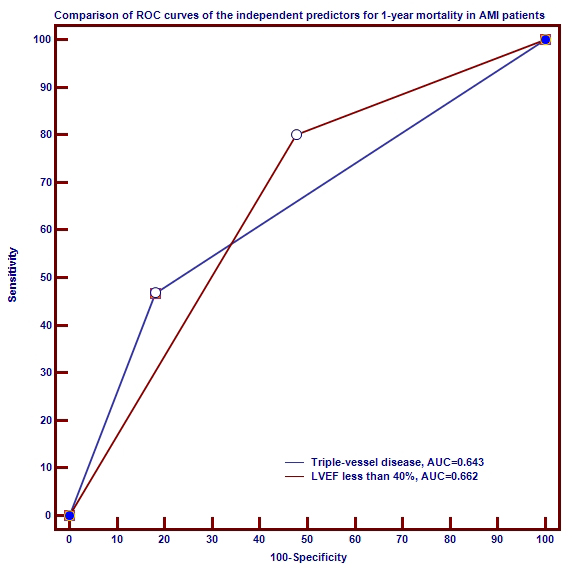 Fig. 4.
Fig. 4.Comparison between ROC curves of the independent prognosticators
for 1-year mortality in AMI patients.
Abbreviations: AMI, acute myocardial infarction; AUC, area under the curve;
LVEF, left ventricular ejection fraction; ROC, receiver operating characteristic.
During the 1-year follow-up phase, 22 patients (8.7%) were rehospitalized. The
relative risk for 1-year readmissions was 2.7 in the octogenarians when compared
with those aged
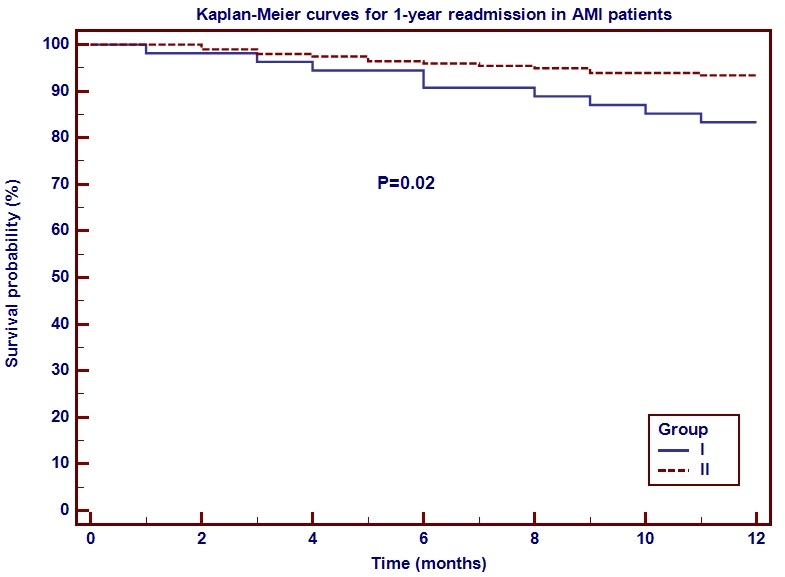 Fig. 5.
Fig. 5.Kaplan-Meier curves for 1-year readmission in AMI patients.
Abbreviations: AMI, acute myocardial infarction.
| Group I | Group II | P value | ||
| Age |
Age |
|||
| n = 51 | n = 200 | |||
| 1-year readmissions | 9 (18%) | 13 (6.5%) | ||
| Causes: | ||||
| Recurrent myocardial infarction | 2 (4%) | 3 (1.5%) | 0.25 | |
| Congestive heart failure | 3 (6%) | 2 (1%) | 0.02 | |
| 3rd Atrio-Ventricular block | 2 (4%) | 3 (1.5%) | 0.25 | |
| Stroke | 1 (2%) | 2 (1%) | 0.55 | |
| Bleeding | 1 (2%) | 3 (1.5%) | 0.80 | |
| Note: Statistically significant values are written in bold (P | ||||
The readmission rate was notably associated in univariate analysis with the
history of CHF (P
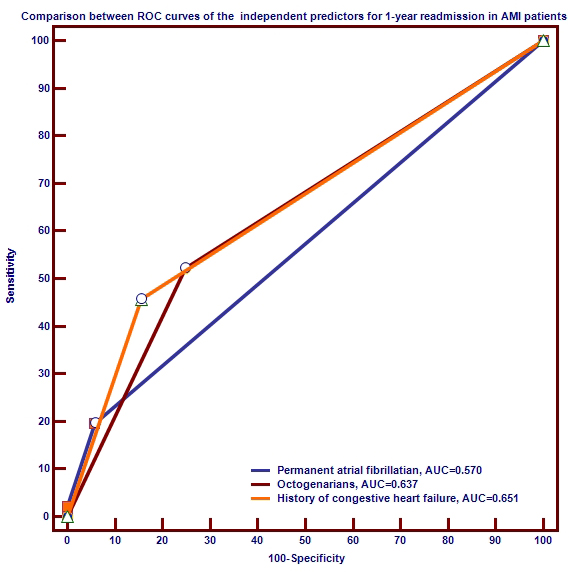 Fig. 6.
Fig. 6.Comparison between ROC curves of the independent prognosticators
for 1-year readmission in AMI patients.
Abbreviations: AMI, acute myocardial infarction; AUC, area under the curve; ROC,
receiver operating characteristic.
During the last decades the incidence of AMI, as well as its mortality, has decreased essentially in developed countries [12, 13]. These favorable tendency reflects a change for the better in many issues that affect the prognosis in patients with AMI [14]. Advanced age, as a circumstance we cannot influence, has a negative prognostic impact value in most studies [15]. One of the most potent variables that improve survival in AMI patients is the myocardial revascularization by PCI [16].
Our study is the first one performed in Romania addressing AMI patients aged
The principal results of the present study were that AMI patients aged
On the subject of the concomitant medication, the diuretics and the oral anticoagulants were more often prescribed in the elderly, as they presented more frequently atrial fibrillationand heart failure, respectively. Although the elderly received more often oral anticoagulants, the in-hospital bleeding rate in the octogenarians was not importantly different from that of the younger AMI patients, probably because the radial approach represents the preferred method of vascular access in our hospital. At discharge, the proportion of patients receiving oral anticoagulants was again higher among the elderly, and their 1-year mortality rate due to bleeding or stroke was higher (P = 0.04). Beside the higher prevalence of oral anticoagulant treatment, group I patients were more often female and had more frequent chronic kidney disease, facts that are increasing the risk factors for bleeding. Advanced age is also related with vascular calcification and fragility, factors that are increasing the bleeding events [19].
A parallel with earlier published statitics is difficult because of the
significantly poorer catheterization and revascularization rates in octogenarian
AMI patients. Mehta et al. [20] estimated in-hospital mortality in STEMI
patients aged
In our study, the 1-year mortality was 12% in the elderly, while the 1-year
readmission rate was 18%. Both the 1-year mortality and the 1-year readmission
rate were significantly higher in the elderly (P
The results from the literature regarding the post-discharge prognosis in the
elderly AMI patients are discordant [22, 23]. In a considerable research, Sui
found that octogenarians have a greater risk of mortality and heart failure
during the first months following the AMI. But, after a few months, the prognosis
of the elderly patients became similar to that of those aged
The current study was an observational cohort study, that included unselected, successive patients with a confirmed diagnosis of AMI admitted in a single center with a readily available catheterization laboratories to perform urgent coronary revascularization. This center provides urgent coronary revascularization for the western region of Romania.
The angiographic results were not evaluated in a blinded manner or by an independent lab, but all angiographies were performed by experienced doctors licensed in interventional cardiology.
We did not use the competing risk analysis, that provides a more interpretable estimate for the survival experience of multiple competing events compared to the traditional Kaplan Meier product-limit method. Although the amount of elderly patients was not great, the event rate was high enough to allow multivariable analyses.
Patients aged
FC and DAB contributed to the conception and design of the study, collected data, wrote and revised the manuscript; AG and BB, revised the manuscript, and insured software and data validation; MCT analyzed the data and supervised the manuscript.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the ethical principles for medical research involving human subjects stated by World Medical Association in the Declaration of Helsinki. The study protocol was approved by the Ethics Committe of Victor Babes University of Medicine and Pharmacy Timisoara, Romania (approval number 2018-0036).
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
