Academic Editor: Takatoshi Kasai
Evaluation of the effects of alirocumab on cardiovascular (CV) events, CV mortality and all-cause mortality. Data search was carried out using the Cochrane Library, PubMed, Web of Science and Embase. The search time is up to November 18, 2020. All randomized clinical trials (AEs) comparing alirocumab with placebo were searched. Meta-analysis was performed by Review Manager version 5.3 (The Cochrane Collaboration, Copenhagen, Denmark), and the heterogeneity between studies was tested by Cochrane’s Q test and measured with I2 statistics. A total of 13 randomized controlled trials with 24,815 participants were included. Alirocumab usage can considerably lower the incidence of CV events when compared to the control group (risk ratio(RR) 0.89, 95% confidence interval(CI) 0.83–0.95). No significant difference in CV mortality between the two groups was observed (RR 0.87, 95% CI 0.74–1.04). Treatment with alirocumab has been associated with a major decrease in all-cause mortality compared to placebo (RR 0.80, 95% CI 0.66–0.96). The incidence of serious adverse events (AEs) was similar in the two groups (RR 0.94, 95% CI 0.90–0.99). Alirocumab can reduce CV events and all-cause mortality. The AEs were mild and tolerable.
Both in Western countries and some Eastern countries, cardiovascular (CV) disease has been the main cause of death in recent years [1, 2]. The level of LDL cholesterol (LDL-C) in plasma is one of the main risk factors for CV [3]. The decrease in LDL-C levels has been reported to be associated with a linear decrease in the risk of CV [4, 5].
Proprotein convertase subtilisin/kexin type 9 (PCSK-9) is a serine protease that promotes the degradation of LDL receptors and decreases the ability of the liver to remove LDL-C from circulation [6, 7]. Alirocumab is a human monoclonal antibody that inhibits human PCSK-9. Alirocumab inhibits hepatic LDLR degradation, increases liver clearance of LDL-C, and reduces plasma LDL-C concentration by binding to circulating PCSK9 [8]. Randomized controlled trial (RCT) data from ODYSSEY results indicate that alirocumab has significant clinical benefits in subjects at high CV risk [9]. However, alirocumab did not reduce the incidence of CV events compared with ezetimibe in the ODYSSEY COMBO II trial. Although meta-analysis has evaluated the effect of PCSK-9 inhibitors on incidence of CV disease and all-cause mortality [10, 11], there is no systematic meta-analysis to directly assess the impact of alirocumab on CV events. Therefore, the evidence for such drugs should be evaluated and synthesized in a timely manner. The purpose of this study is to systematically review and compile all available Alirocumab RCTs in order to assess their effect on CV events, CV mortality, all-cause mortality and adverse events (AEs).
This study was performed in accordance with the PRISMA (Preferred Reporting of Systematic Reviews and Meta-Analysis) (Supplementary Table 1).
The PubMed, Cochrane Library, Web of Science and Embase databases were searched until November 18, 2020. The search terms were searched using keywords and Mesh terms, as shown below: alirocumab, SAR236553, REGN-727, randomized controlled trials and clinical trials, etc. We also searched manually for references to included studies and current systematic reviews to identify any other publications that could be potentially relevant. The search is limited to English.
The inclusion criteria were as follows: (1) RCTs published in peer-reviewed journals; (2) Comparison of Alirocumab and control (placebo or alternative lipid-lowering therapy); (3) Report data on CV events, CV mortality, all-cause mortality or AEs.
The Reviews, reports, non-randomized studies, and studies using other anti-PCSK9 antibodies (e.g., evolocumab, bococizumab) were excluded.
Two investigators used the prespecified data collection form to extract data independently. The following data were mainly extracted: first author, publication year, number of participants and average age range, treatment, participant characteristics, intervention type, median follow-up time, and relevant data on outcome indicators. The third author cross-checked all entries and arbitrated for any inconsistencies. Besides, the risk of bias in the included trials was evaluated using the Cochrane Collaboration’s method.
Meta-analysis was conducted using Review Manager version 5.3 (The Cochrane
Collaboration, Copenhagen, Denmark). The risk ratio (RR) was used for dichotomous
variables, and the variables were expressed in 95% confidence intervals (CI).
The heterogeneity of each outcome in the trial was estimated by
Fig. 1 shows the document screening flow chart of this study. A total of 1651 articles were obtained in the initial literature search. After the screening of the titles and abstracts, 25 studies met the criteria for a full-text review. Following the screening of the titles and abstracts, 25 papers were qualified for a full text analysis and 13 of them were included in the final review [9, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23]. Thirteen studies included 24,815 patients, of which 13,245 were allocated to the alirocumab intervention and 11,570 were allocated to the control group. In the included trials, patients took 75 mg of alirocumab every 2 weeks and then increased to 150 mg every 2 weeks partway throughout the trial based on the LDL-C response. The duration of the intervention ranged between 24 weeks and 146 weeks. The average age was between 51.7 and 64.6 years, and 72.3% of the patients are male. Baseline patient characteristics for the study arms included were shown in Table 1 (Ref. [9, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23]). The overall quality of included studies was moderate, and the risk of bias for most items was low (Fig. 2).
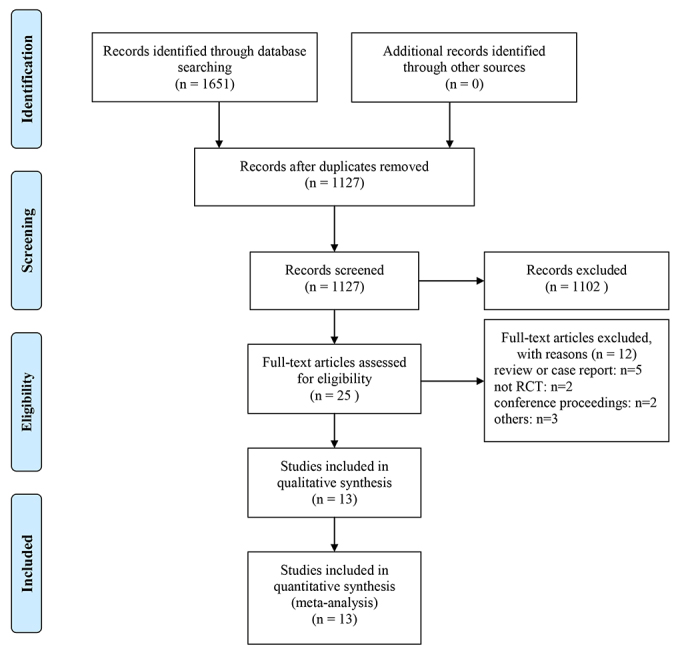 Fig. 1.
Fig. 1.Flow diagram showing the study selection process for the meta-analysis. The number of studies shown at the bottom of the flow chart represents studies that were ultimately considered eligible for inclusion in this meta-analysis.
 Fig. 2.
Fig. 2.Risk of bias assessment in randomized controlled trials (RCTs). (A) The risk of bias summary of the included RCTs. Green represents low risk, yellow unclear risk, and red high risk. (B) The risk of bias graph of the included RCTs. Green represents low risk, yellow unclear risk, and red high risk.
| Study | Intervention | N | Male | Age, y mean (SD) or median (range) | Body mass index (kg/m |
Patients | Background treatment | Follow-up duration, weeks |
| Cannon 2015 [12] | Alirocumab 75/150 Q2W | 479 | 360 | 61.7 |
30.0 |
hypercholesterolaemia and established CHD | a maximally tolerated dose of statin | 104 |
| Ezetimibe | 241 | 170 | 61.3 |
30.3 |
or CHD risk-equivalents | |||
| Kastelein FH I 2015 [15] | Alirocumab 75/150 Q2W | 323 | 180 | 52.1 |
29.0 |
HeFH | stable high dose statin | 88 |
| Placebo | 163 | 94 | 51.7 |
30.0 | ||||
| Kastelein FH II 2015 [15] | Alirocumab 75/150 Q2W | 167 | 86 | 53.2 |
28.6 |
HeFH | stable high dose statin | 88 |
| Placebo | 82 | 45 | 53.2 |
27.7 | ||||
| Kereiakes 2015 [16] | Alirocumab 75/150 Q2W | 209 | 131 | 63.0 |
32.62 |
high CV risk | a stable, maximally tolerated statin dose | 24 |
| Placebo | 107 | 77 | 63.0 |
32.03 | ||||
| Moriarty 2015 [18] | Alirocumab 75/150 Q2W | 126 | 70 | 64.1 |
29.6 |
moderate or high CV risk | no statin/stable dose of statin | 24 |
| Ezetimibe | 125 | 67 | 62.8 |
28.4 | ||||
| Robinson 2015 [19] | Alirocumab 75/150 Q2W | 1553 | 983 | 60.4 |
30.2 |
LDL-C |
a maximally tolerated dose of statin | 78 |
| Placebo | 788 | 474 | 60.6 |
30.5 | ||||
| Ginsberg 2016 [13] | Alirocumab 75/150 Q2W | 72 | 35 | 49.8 |
28.8 |
HeFH and LDL-C |
a maximally tolerated stable dose of statin | 88 |
| Placebo | 35 | 22 | 52.1 |
28.9 | ||||
| Roth 2016 [20] | Alirocumab 75 Q2W | 37 | 14 | 59.3 |
29.7 |
hypercholesterolemia at moderate-to-very- | a maximally tolerated dose of statin | 24 |
| Placebo | 73 | 40 | 59.4 |
32.3 |
high CV risk | |||
| Stroes 2016 [21] | Alirocumab 75 Q2W | 116 | 69 | 62.5 |
29.4 |
hypercholesterolemia | no statin | 24 |
| Placebo | 58 | 31 | 63.1 |
28.5 | ||||
| Teramoto 2016 [23] | Alirocumab 75/150 Q2W | 144 | 84 | 60.3 |
25.6 |
HeFH or non-HeFH at high CV risk | stable dose of statin | 52 |
| Placebo | 72 | 47 | 61.8 |
25.4 | ||||
| Koh 2018 [17] | Alirocumab 75 Q2W | 97 | 83 | 61.2 |
26.3 |
hypercholesterolemia at high cardiovascular risk | stable dose of statin | 24 |
| Placebo | 102 | 81 | 60.1 |
26.6 | ||||
| Schwartz 2018 [9] | Alirocumab 75 Q2W | 9462 | 7072 | 58.5 |
28.5 |
recent ACS and inadequate lipid control | maximum tolerated dose of of statin | 146 |
| Placebo | 9462 | 7090 | 58.6 |
28.5 | ||||
| Teramoto 2019 [22] | Alirocumab 150 Q2W | 53 | 33 | 63.6 |
26.4 |
HeFH or non-FH | the lowest-strength dose of | 12 |
| Placebo | 56 | 37 | 64.6 |
25.6 |
atorvastatin or a non-statin therapy | |||
| Han 2020 [14] | Alirocumab 75/150 Q2W | 407 | 315 | 58.8 |
25.6 |
hypercholesterolemia at high CV risk | a maximally tolerated statin dose | 24 |
| Ezetimibe | 206 | 146 | 58.3 |
25.2 |
The CV events analysis included 13 RCTs (including 24,803 patients). Alirocumab
treatment can dramatically lower the incidence of CV events when compared to the
control group (10.9% versus 13.4%; RR 0.89, 95% CI 0.83–0.95; I
 Fig. 3.
Fig. 3.Association of alirocumab compared with placebo with the incidence of CV events. Each square shows effect estimate of individual studies with their 95% CI. Size of squares is proportional to the weight of each study in the meta-analysis. In this plot, studies are shown in the order of publication date and first author’s names.
The incidence of CV mortality was reported in four RCTs (including 20,570
patients). There was no significant difference in CV mortality between alirocumab
and the control groups (2.3% versus 2.7%; RR 0.87, 95% CI 0.74–1.04; I
 Fig. 4.
Fig. 4.Association of alirocumab compared with placebo with the incidence of CV mortality. Each square shows effect estimate of individual studies with their 95% CI. Size of squares is proportional to the weight of each study in the meta-analysis. In this plot, studies are shown in the order of publication date and first author’s names.
A total of 13 studies (including 24,773 patients) were included in the analysis
of all-cause mortality. Compared with placebo, alirocumab can reduce the
all-cause mortality of patients (1.6% versus 2.1%; RR 0.80, 95% CI 0.66–0.96;
I
 Fig. 5.
Fig. 5.Association of alirocumab compared with placebo with the incidence of all-cause mortality. Each square shows effect estimate of individual studies with their 95% CI. Size of squares is proportional to the weight of each study in the meta-analysis. In this plot, studies are shown in the order of publication date and first author’s names.
Thirteen studies were included in the analysis of SAEs, including 24,748
patients. A substantial 2.2% decrease in the occurrence of severe AEs (SAEs) was
observed in the alirocumab group relative to the control group (RR 0.94, 95% CI
0.90–0.99; I
 Fig. 6.
Fig. 6.Forest plots showing the effect of clinical outcomes on the SAEs and AEs. (A) Association of alirocumab compared with placebo with the incidence of SAEs. (B) Association of alirocumab compared with placebo with the incidence of AEs. Each square shows effect estimate of individual studies with their 95% CI. Size of squares is proportional to the weight of each study in the meta-analysis. In this plot, studies are shown in the order of publication date and first author’s names.
Funnel plot symmetry evaluation did not show any signs of publication bias for CV events, all-cause mortality or safety (Fig. 7). The sensitivity analysis omitted one study at a time and did not yield different results in terms of CV events, CV mortality, all-cause mortality, and AEs (Supplementary Fig. 1).
 Fig. 7.
Fig. 7.Funnel plot of CV events. (A), all-cause mortality (B), SAEs (C) and AEs (D). Funnel plot of MACE depicting the publication bias of CV events, all-cause mortality, SAEs and AEs between alirocumab and placebo groups. Funnel plot is symmetrical distribution, which represents a low publication bias.
Alirocumab is a human monoclonal antibody against PCSK-9. It is administered by subcutaneous injection every 2 weeks. The initial dose is 75 mg, the dose can be increased to 150 mg if LDL-C is not sufficiently reduced. In this study, a meta-analysis of RCTs was undertaken to examine the effect of treatment with alirocumab vs. placebo on CV events, CV mortality, all-cause mortality and SAEs. However, no difference was observed in CV mortality. The results of this meta-analysis are basically consistent with the results of the previous meta-analysis that evaluated PCSK-9 inhibitor [10, 11].
In the ODYSSEY experiment, no difference was reported in CV mortality or
all-cause death or SAEs, except that the composite primary endpoint was
significantly reduced [9]. The study found that patients who received alirocumab
had a lower risk of CV events compared with patients who received placebo.
Moreover, alirocumab can significantly reduce the risk of CV events in patients
with baseline LDL cholesterol
However, it has been reported that a delay between the effect on LDL-C levels and the appearance of clinical benefit has been observed in clinical trials of statins [10]. Furthermore, it was also observed that the extent of the risk mitigation of the secondary tended to increase over time in the FOURIER and ODYSSEY OUTCOMES studies [9, 25]. These results indicate that it takes time to convert LDL-C reduction into CV benefits, and the longer the follow-up time, the more obvious the benefits of CV events observed.
Although there is no significant difference in CV mortality between the alirocumab and control groups, alirocumab can significantly lower all-cause mortality, which may be connected to the risk of non-CV mortality. It is reported that alirocumab reduces non-fatal cardiovascular events and may have an impact on non-cardiovascular and cardiovascular deaths, which in turn affects all-cause deaths [26]. Despite this, current research is divided on whether alirocumab will lower all-cause mortality [10, 27, 28]. Previous research has revealed that statin medication required 5 to 6 years of exposure to observe the advantages of cholesterol-lowering treatment on mortality [29]. Therefore, additional and longer follow-up trials may be required to determine the advantages of alirocumab on all-cause mortality.
Regarding safety, this study showed that alirocumab had mild AEs and all-cause mortality was significantly lower than placebo. The incidence of SAEs was similar between alirocumab and placebo group. There was no statistically significant difference in the incidence of AEs between alirocumab and placebo.
This study presents an analysis on a topic on which numerous papers have already been published. The study analyzed CV events and CV mortality, and included all clinical trials published thus far. However, this study has some limitations. First of all, the follow-up time of the included studies is too short. Most studies have a follow-up time of 12 or 24 weeks. The long-term effect is not apparent. Secondly, ODYSSEY OUTCOMES contributed more than 85% of the data for all results and strongly influenced all effect estimates.
In conclusion, alirocumab therapy can effectively reduce the incidence of CV events and all-cause mortality. There was no significant difference in the incidence of AE in the alirocumab group compared to the control group. However, the impact of alirocumab on CV mortality is still inconclusive, and more long-term studies are needed to solve this issue.
WWT—extraction, analysis and interpretation of data, drafting of the manuscript; FZQ—analysis of data, manuscript revision; BJH—design and revision, statistical analysis; All—final approval of the manuscript submitted.
Not applicable.
Thanks to everyone who contributed to this study.
This research received no external funding.
The authors declare no conflict of interest.
