† These authors contributed equally.
Academic Editor: Leonardo De Luca
Older age is known as a negative prognostic parameter in patients with acute
myocardial infarction (AMI). In this study, we aimed to investigate age-related
differences in treatment protocols, in-hospital and 1-year mortality. This
retrospective observational single-center study enrolled consecutive AMI patients
with an urgent percutaneous coronary intervention (PCI) as the main method of
myocardial revascularization. The patients divided were divided by age into group
I (
Subjects aged
Standard therapies are not always applied in the elderly, as the evidence of benefit is deficient and the danger of complications is high for this age group [1, 2, 3]. These facts can be also explained by some specific clinical characteristics of the elderly at presentation: the symptoms of the ACS may be atypical, the electrocardiographic signs are often less specific and the comorbidities may lead to a confounding clinical picture. All these may lead to diagnostic incertitude and postponed or conservative therapeutic strategies [1].
This situation can be found in Romania too, though aggravated by the small
number of catheterization laboratories that are able to perform urgent coronary
revascularization (23, for a population of 20 million inhabitants). Our
retrospective study is the first one done in Romania addressing AMI patients aged
This is a retrospective cohort study. Between 1 January and 31 December of 2020, 524 patients with AMI were admitted to the Cardiology Clinic of the Timisoara Institute of Cardiovascular Diseases within the first 12 hours of the onset of the symptoms. In the absence of contraindications, urgent percutaneous coronary intervention (PCI) was done.
The initial evaluation was grounded on the analysis of low and/or
high-probability features resulting from symptoms and signs at presentation,
12-lead ECG, and cardiac troponin [4, 5]. The diagnosis of STEMI was grounded on
the existence of at minimum 2 of these 3 parameters: (1) typical angina lasting
for more than 20 minutes; (2) ST-segment elevation
The PCI was performed and the associated pharmacological treatment was administered according to the European Society of Cardiology (ESC) guidelines [4, 5]. All patients were given a loading dose of 300–600 mg clopidogrel. They habitually received before PCI 5000 IU unfractionated heparin and 300–500 mg aspirin. Glycoprotein IIb/IIIa inhibitors were administered when the operator considered it necessary. If a coronary stent was implanted, clopidogrel was prescribed for 12 months, associated with aspirin.
The inclusion criteria were a confirmed diagnosis of AMI in patients hospitalized within the first 12 hours of the symptoms onset and the absence of exclusion criteria.
Exclusion criteria were: PCI‑related or CABG-related AMI, the presence of diseases worsening the long‑term prognosis such as severe primary cardiomyopathy, severe valvular diseases or congenital heart diseases, kidney dysfunction, liver cirrhosis, a malignant tumor, and severe infection.
The study was advised by the Ethics Commission of the Victor Babes” University of Medicine and Pharmacy. All patients provided written informed consent for participation in the study, in accordance with the Human Rights Declaration of Helsinki.
Baseline data were taken from hospital records and comprised gender, age, Killip functional class on admission, medical history, 12 leads resting electrocardiogram, laboratory data, echocardiographic data, and the results of the coronary angiography.
Medical history integrated information about smoking status, obesity, diabetes, old myocardial infarction, history of stroke, hypertension, peripheral artery disease, chronic obstructive pulmonary disease, chronic kidney disease. The cardiac biomarkers determined at admission were: MB fraction of creatine kinase (CK) and cardiac troponin levels. Further laboratory records were: blood cell count, serum hemoglobin, serum glucose, serum creatinine, estimated glomerular filtration, serum electrolytes, and lipogram.
Medical treatment reports were accomplished at discharge and at the 1-year follow-up.
The cause of death was determined from hospital records, or by a phone conversation with the patient‘s physician for those who died at home.
All causes of readmissions were noted during the 1-year follow-up period. The causes of readmissions were determined by utilizing the hospital records.
The primary endpoint was in-hospital mortality, stated as the death of any cause in the course of the hospitalization for AMI. As cardiac deaths were regarded as those due to AMI, heart failure, cardiogenic shock, acute pulmonary edema, cardiac rupture, or ventricular fibrillation. Noncardiac deaths were stated as deaths having an extra-cardiac cause, e.g., stroke, sepsis, acute renal failure.
The secondary endpoints were mortality and readmission rates throughout the 1-year follow-up phase. 1-year mortality included all-cause (cardiac and non-cardiac) deaths. 1-year readmissions included as causes recurrent myocardial infarction (MI), stent thrombosis, stroke, and bleeding. Recurrent MI was defined using the Academic Research Consortium criteria [6]. Bleeding complications were stated using the Bleeding Academic Research Consortium and the Thrombolysis In Myocardial Infarction bleeding classifications [7, 8]. Stroke was diagnosed in the presence of an irreversible neurological deficit, as stated by a neurologist, and based on supporting evidence, such as brain images.
Important coronary stenosis was stated when a reduction in the internal diameter of at least 75% in the anterior descending, circumferential or right coronary artery and at least 50% in the left main coronary trunk was seen. Multivessel coronary artery disease was stated when important stenosis in several coronary arteries was documented [4, 5].
PCI-related AMI (type 4) was defined as an AMI occurring
CABG-related AMI (type 5) was defined as an AMI occurring
A patient was stated to be hypertensive when his blood pressure was
Echocardiographic examination was performed during the first 24 hours of hospital admission, using a VIVID S5 ultrasonograph device. LVEF was calculated using the Simpson method. The E/A ratio was determined by means of the antegrade mitral flow [17].
We used the classification the Killip classification of heart failure severity in AMI patients [18].
Data were collected and analyzed using the MedCalc Statistical Software version
19.1.7 (MedCalc Software Ltd, Ostend, Belgium) for Windows. Data are given as
mean
Of the 522 admitted with ACS, 476 were registered in the research. The mean age
was 67.38
| Group I | Group II | P value | |
| Age |
Age | ||
| n = 264 | n = 212 | ||
| Mean age, years (X ± 1 SD) | 75.9 ± 7.2 | 53.5 ± 8 | |
| Male sex (n, %) | 135 (51%) | 159 (75%) | |
| Smokers (n, %) | 91 (34%) | 121 (57%) | |
| Obesity (n, %) | 66 (25%) | 53 (25%) | 1 |
| Diabetes mellitus (n, %) | 77 (29%) | 53 (25%) | 0.33 |
| Hypercholesterolemia (n, %) | 185 (70%) | 153 (72%) | 0.63 |
| COPD (n, %) | 79 (30%) | 53 (25%) | 0.22 |
| Chronic kindey disease (n, %) | 50 (19%) | 23 (11%) | 0.01 |
| Systemic hypertension (n, %) | 224 (85%) | 161 (76%) | 0.01 |
| Peripheral artery disease (n, %) | 20 (7.4%) | 8 (4%) | 0.11 |
| History of stroke (n, %) | 55 (21%) | 28 (13%) | 0.02 |
| Old myocardial infarction (n, %) | 32 (12%) | 19 (9%) | 0.29 |
| Previous PCI (n, %) | 21 (8%) | 11 (5%) | 0.18 |
| Previous CABG (n, %) | 5 (2%) | 2 (0.8%) | 0.27 |
| Known congestive heart failure | 63 (24%) | 27 (12.5%) | |
| STEMI (n, %) | 230 (87%) | 201 (95%) | 0.003 |
| NSTEMI (n, %) | 34 (13%) | 11 (5%) | 0.003 |
| Killip class at admission | 2.4 ± 1 | 2.1 ± 0.9 | 0.0007 |
| Heart rate at admission (X ± 1 SD) | 80.5 ± 19.9 | 80.2 ± 18 | 0.86 |
| Systolic BP at admission (X ± 1 SD) | 128 ± 27 | 131 ± 26 | 0.35 |
| Diastolic BP at admission (X ± 1 SD) | 73.5 ± 17.1 | 77.6 ± 17.1 | 0.05 |
| Atrial Fibrillation at admission (n, %) | 62 (23.3%) | 23 (11%) | 0.0005 |
| - acute (n, %) | 32 (12.3%) | 14 (6.6%) | 0.03 |
| - persistent (n, %) | 30 (11%) | 9 (4.4%) | 0.008 |
| Recenl LBBB at admission (n, %) | 11 (4%) | 4 (1.7%) | 0.14 |
| AV block at admission (n, %) | 26 (10%) | 4 (1.7%) | 0.0002 |
| - 2nd degree (n, %) | 5 (2%) | 2 (0.09%) | 0.05 |
| - 3rd degree (n, %) | 21 (8%) | 12 (0.09%) | |
| Ventricular fibrillation at admission (n, %) | 24 (9%) | 14 (6.6%) | 0.56 |
| LVEF at admission |
153 (58%) | 87 (41%) | 0.0002 |
| E/A ratio at admission |
182 (69%) | 119 (56%) | 0.003 |
| Scr ( |
103.75 |
75.79 |
|
| BNP (pg/mL, mean |
881.69 |
834.79 |
0.04 |
| CK-MB |
50.01 |
51.34 |
0.38 |
| Tpn‑I ( |
14.98 |
14.69 |
0.165 |
| Note: Statistically significant values are shown in bold (P Abbreviations: AMI, acute myocardial infarction; STEMI, acute myocardial infarction woth persistent ST-segment elevation; NSTEMI, acute myocardial infarction without ST-segment elevation; BP, blood pressure; LBBB, left ventricular blood pressure; AV, atrio-ventricular; PCI, Percutaneous coronary intervention; CABG, Coronary artery bypass grafting; LVEF, Left ventricular ejection fraction; E/A—the ratio of peak velocity blood flow in early diastole to peak velocity flow in late diastole; Scr, Serum creatinine; BNP, Brain natriuretic peptide; CK‑MB, Creatine kinase‑MB; Tpn‑I, Troponin‑I; LWWH, Low molecular weight heparin; ACEI, Angiotensin‑converting enzyme inhibitor; ARB, Angiotensin receptor blocker; CCB, Calcium antagonists. | |||
Table 2 presents the data of emergency coronagraphy. No angiography could be done in 5 (1.9%) of group I patients and 2 (0.09%) patients of group II (P = 0.66), because of severe kidney failure. Group II patients had a significantly higher proportion of monovascular coronary disease (P = 0.0001), a significantly lower proportion of triple vessel disease (P = 0.03), and had a significantly higher rate of interventional revascularization by PCI (79% vs. 65%, (P = 0.0008). The rate of coronary artery bypass graft was 2.6% in group II and 2% in group I (P = 0.66). Regarding the concomitant medication, diuretics were more often administered in group I patients (P = 0.02).
| Group I | Group II | P value | |
| Age |
Age | ||
| n = 264 | n = 212 | ||
| No angiography performed | 5 (1.9%) | 2 (0.09%) | 0.05 |
| Angiographic findings | |||
| - Single vessel disease | 100 (38%) | 119 (56%) | 0.0001 |
| - Dual vessel disease | 55 (21%) | 36 (17%) | 0.27 |
| - Triple vessel disease | 66 (25%) | 36 (17%) | 0.03 |
| - Left main disease | 40 (15%) | 21 (10%) | 0.10 |
| Interventional revascularization: | 177 (67%) | 174 (82%) | 0.0002 |
| - PCI | 172 (65%) | 168 (79%) | 0.0008 |
| - CABG | 5 (2%) | 6 (2.6%) | 0.66 |
| Concomitant drug therapy | |||
| - Clopidogrel | 254 (96.3) | 209 (98.7%) | 0.10 |
| - Aspirin | 256 (97.2%) | 210 (99.2%) | 0.11 |
| - LMWH | 2145 (92.9%) | 203 (95.9%) | 0.16 |
| - Betablockers | |||
| - Statin | 200 (76.1%) | 72 (81.3%) | 0.17 |
| - ACEI/BRA | 251 (95.2%) | 206 (97.2%) | 0.26 |
| - CCB | 190 (72.2%) | 156 (73.8%) | 0.69 |
| - Diuretics | 93 (35.3%) | 65 (30.5%) | 0.27 |
| 94 (35.5%) | 54 (25.6%) | 0.02 | |
| Note: Statistically significant values are shown in bold (P Abbreviations: AMI, acute myocardial infarction; STEMI, acute myocardial infarction woth persistent ST-segment elevation; NSTEMI, acute myocardial infarction without ST-segment elevation; BP, blood pressure; LBBB, left ventricular blood pressure; AV, atrio-ventricular; PCI, Percutaneous coronary intervention; CABG, Coronary artery bypass grafting; LVEF, Left ventricular ejection fraction; E/A—the ratio of peak velocity blood flow in early diastole to peak velocity flow in late diastole; Scr, Serum creatinine; BNP, Brain natriuretic peptide; CK‑MB, Creatine kinase‑MB; Tpn‑I, Troponin‑I; LWWH, Low molecular weight heparin; ACEI, Angiotensin‑converting enzyme inhibitor; ARB, Angiotensin receptor blocker; CCB, Calcium antagonists. | |||
The total all-cause mortality rate, including in-hospital and 1-year mortality, was 14.3% (n = 67). The number of deaths was 50 (18.9%) for group I and 17 (8%) for group II, P = 0.0004.
During the hospitalization for AMI, 53 patients (12.3%) died, 39 being from
group I (20%), and 14 from group II (6%), P
 Fig. 1.
Fig. 1.Kaplan-Meier curves for in-hospital mortality.
| Group I | Group II | P value | ||
| Age |
Age | |||
| n = 264 | n = 212 | |||
| Total mortality n = 67 (14.3%) | 50 (18.9%) | 17 (8%) | 0.0004 | |
| In hospital mortality n = 53 (12.3%) | 39 (20%) | 14 (6%) | ||
| Cardiac causes n = 44 (9.2%) | 32 (12%) | 12 (5.6%) | 0.016 | |
| - Ventricular fibrillation | 14 (5%) | 5 (2.3%) | 0.12 | |
| - Electromechanical dissociation | 5 (1.8%) | 2 (0.9%) | 0.40 | |
| - Cardiogenic shock | 10 (3.7%) | 3 (1.4%) | 0.12 | |
| - Acute pulmonary edema | 3 (1.1%) | 2 (0.9%) | 0.82 | |
| Noncardiac causes n = 9 (1.8%) | 7 (2.6%) | 2 (0.9%) | 0.17 | |
| - Acute renal failure | 3 (1.1%) | 1 (0.9%) | 0.83 | |
| - Bleeding | 2 (0.7%) | 1 (0.9%) | 0.80 | |
| - Stroke | 1 (0.3%) | - | ||
| - Sepsis | 1 (0.3%) | - | ||
| Discharged patients n = 423 | Group I | Group II | P value | |
| Age |
Age | |||
| n = 225 | n = 198 | |||
| Medication at discharge | ||||
| Clopidogrel | 180 (80%) | 168 (85%) | 0.17 | |
| Aspirin | 193 (86%) | 167 (84%) | 0.56 | |
| Betablockers | 143 (64%) | 144 (73%) | 0.04 | |
| Statin | 182 (81%) | 170 (86%) | 0.16 | |
| ACEI/BRA | 161 (72%) | 154 (78%) | 0.15 | |
| Oral anticoagulants | 62 (26%) | 23 (12%) | 0.0003 | |
| CCB | 90 (40%) | 61 (31%) | 0.05 | |
| Diuretics | 80 (35%) | 52 (26%) | 0.045 | |
| 1-year mortality n = 14 (3.3%) Causes: | 11 (4.8%) | 3 (1.5%) | 0.05 | |
| Recurrent myocardial infarction | 3 (1.3%) | 2 (1%) | 0.77 | |
| Congestive heart failure | 5 (2.2%) | 1 (0.5%) | 0.13 | |
| Stroke | 2 (0.9%) | - | ||
| Bleeding | 1 (0.4%) | - | ||
| Note: Statistically significant values are shown in bold (P Abbreviations: ACEI, Angiotensinconverting enzyme inhibitor; ARB, Angiotensin receptor blocker; CCB, Calcium antagonists. | ||||
The relative risk for in-hospital death for Group I patients was 2.5 (95% CI 1.12–5.77), P = 0.001, and 0.39 for Group II patients (95% CI 0.17–0.88).
The multivariate logistic regression selected two parameters as independent
predictors for in-hospital death. These parameters were age
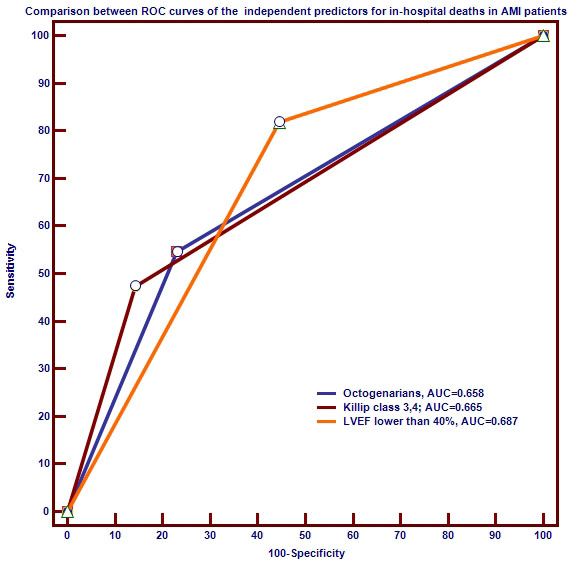 Fig. 2.
Fig. 2.Comparison of receiver operating characteristic (ROC) curves of independent variables predictive for in-hospital all-cause death risk.
423 AMI patients were discharged alive (88.8%). Group I patients received at discharge more often oral anticoagulants (P = 0.0003) and diuretics (P = 0.045). and less often beta-blockers (P = 0.04).
During the 1-year follow-up phase, further 14 patients died. The 1-year mortality was 3.3%, slightly higher in group I patients (4.8% vs. 1.5%, P = 0.05), with no notable differences among the causes of death (Table 3).
Throughout the 1-year follow-up phase, 22 patients (5.2%) were rehospitalized. The readmission rate was lower in group I patients, but the difference was not notable (P = 0.24). The causes of readmissions had similar frequencies in the two groups (Table 4).
| Group I | Group II | P value | ||
| Age |
Age | |||
| n = 225 | n = 198 | |||
| 1-year readmissions | ||||
| n = 22 (5.2%) | 9 (4%) | 13 (6.5%) | 0.24 | |
| Causes: | ||||
| Recurrent myocardial infarction | 2 (0.9%) | 3 (1.5%) | 0.56 | |
| Congestive heart failure | 3 (1.3%) | 2 (1%) | 0.77 | |
| 3rd Atrio-Ventricular block | 2 (0.9%) | 3 (1.5%) | 0.56 | |
| Stroke | 1 (0.4%) | 2 (1%) | 0.45 | |
| Bleeding | 1 (0.4%) | 3 (1.5%) | 0.23 | |
| Note: Statistically significant values are shown in bold (P | ||||
In Romania, cardiovascular diseases are responsible for 63% of all deaths, while in Europe the proportion is 37%. AMI represents the main cause of death in patients with coronary artery disease. In our country, about 13,000 people experience an AMI every year, and the AMI-related death rates reach disturbing levels. The death risk is greatest during the first 2 hours from the onset of the symptoms. According to the data published by the Romanian Registry for ST-Elevation Myocardial Infarction (RO-STEMI), 52% of deaths take place before the patient reaches the hospital. The death rate drops considerably after admission, reaching 19% on the first day and 8% on the second day of hospitalization. About 21% of deaths occur later on, up to 1 month after the AMI [19].
During the last decades the incidence of AMI, as well as its mortality, has decreased essentially in developed countries [20, 21]. This favorable tendency reflects a change for the better in many parameters that affect the prognosis in patients with AMI. Advanced age, as a parameter we cannot influence, has a negative prognostic impact value in most studies [22]. One of the most potent variables that improve outcomes in AMI patients is the myocardial revascularization by urgent PCI [23]. We applied the standard WHO definition of elderly patients [24].
Our study is the first one performed in Romania addressing AMI patients aged
All patients were treated at an academic tertiary hospital, able to provide 24/7
catheterizations and to ensure the urgent coronary revascularization
interventions for the western region of Romania. All reperfusion procedures were
done only by PCI, in a group of successive, unselected AMI patients, with or
without ST-segment elevation. The main findings of the present observational
cohort study were as follows: patients with AMI
Despite the added, recognized risk factors and the poorer expected outcome in
the elderly AMI patients, we found that the rate of diagnostic coronary
angiography of was notably lower in this high-risk group (67% vs. 82%,
P
At discharge, the elderly patients received more often diuretics and oral anticoagulants, and less often beta-blockers. Among the discharged patients, mortality during the 1-year follow-up period was marginally higher in the elderly (P = 0.05). The readmission rates were similar (P = 0.25) in the two patient groups After discharge, the survivors were followed up for 1 year.
An assessment in relationship with other previously published data in Romania is
difficult due to the notably lower catheterization and revascularization rates in
the elderly AMI patients, in our country. Mehta et al. [25] evaluated
in-hospital mortality in STEMI patients (age
In our study, the 1-year mortality was 4.8% in the AMI patients
The current study was observational, non-randomized, conducted in a single center, with no control group. Nonetheless, the research included unselected, successive AMI patients. AMI admitted to a center with readily available catheterization laboratories to perform urgent coronary revascularization, which provides urgent coronary revascularization for the western region of Romania.
Although the angiographic findings were not evaluated by an independent laboratory or in a blinded mode, although, they were assessed by qualified physicians with great experience in interventional cardiology.
AMI patients aged
FC and DAB contributed to the conception and design of the study, collected data, wrote and revised the manuscript; MCT and IMC analysed the data and supervised the manuscript.
The study was advised by the Ethics Commission of the Victor Babes” University of Medicine and Pharmacy (Ethics approval number: 020). All patients provided written informed consent for participation in the study, in accordance with the Human Rights Declaration of Helsinki.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
