Academic Editor: Peter A. McCullough
We evaluated the age-specific mortality of unselected adult outpatients infected
with SARS-CoV-2 treated early in a dedicated COVID-19 day hospital and we
assessed whether the use of hydroxychloroquine (HCQ) + azithromycin (AZ) was
associated with improved survival in this cohort. A retrospective monocentric
cohort study was conducted in the day hospital of our center from March to
December 2020 in adults with PCR-proven infection who were treated as outpatients
with a standardized protocol. The primary endpoint was 6-week mortality, and
secondary endpoints were transfer to the intensive care unit and hospitalization
rate. Among 10,429 patients (median age, 45 [IQR 32–57] years; 5597 [53.7%]
women), 16 died (0.15%). The infection fatality rate was 0.06% among the 8315
patients treated with HCQ+AZ. No deaths occurred among the 8414 patients younger
than 60 years. Older age and male sex were associated with a higher risk of
death, ICU transfer, and hospitalization. Treatment with HCQ+AZ (0.17
[0.06–0.48]) was associated with a lower risk of death, independently of age,
sex and epidemic period. Meta-analysis evidenced consistency with 4 previous
outpatient studies (32,124 patients—Odds ratio 0.31 [0.20–0.47], I
The SARS-CoV-2 pandemic infected 200 million people and killed 4.2 million people by August 4, 2021, corresponding to an overall infection fatality rate (IFR) of 2% [1]. Health agencies in Western countries have focused on contagion control measures (lockdown), late-stage hospitalized patients, intensive care units, and vaccination, but for reasons that are yet to be clarified, early treatment has not been emphasized [2, 3, 4]. In eastern countries such as China, India, Iran, and Saudi Arabia, where early treatment and prevention with repurposed antivirals, particularly hydroxychloroquine (HCQ), has been widely implemented [5, 6, 7, 8], lower IFRs than Western countries, where early treatment with orally available molecules has been overlooked or even discouraged, have been reported [1]. In addition, countries using chloroquine or HCQ as a treatment from the start of the epidemic had a much slower dynamic in daily deaths [9]. The antiviral effect of chloroquine and its derivatives (HCQ) against SARS-CoV-2 was identified as early as February 2020 through in vitro studies in early Chinese publications [10, 11] and a preliminary trial in our center [12]. The synergistic in vitro antiviral effect of the combination of HCQ with azithromycin (AZ) was further reported [13]. In addition, HCQ has several anti-inflammatory and antithrombotic properties [14], which is of particular interest in the context of COVID-19-associated inflammation and coagulopathy.
Our previous observational study [15] reported a beneficial effect on thousands of cases, but in- and outpatients were not analyzed separately. The largest publicly available ambulatory studies included an Iranian study with 28,759 outpatients and a study in Saudi Arabia with 5541 outpatients, both evidencing a 4-fold reduced risk of death with HCQ [5, 6]. The importance of earliness of treatment has also been recently emphasized by a Chinese study reporting that HCQ, when administered in the first 5 days after symptom onset, improves prognosis and reduces viral shedding [7]. The effect of early ambulatory treatment with HCQ combined with AZ on COVID-19 mortality has not been reported in a large series. Here, we evaluated the age-specific mortality of unselected adult outpatients infected with SARS-CoV-2 managed early in a dedicated COVID-19 day hospital offering standardized treatment based on HCQ+AZ. We also assessed whether the use of HCQ+AZ was associated with improved IFR and lower rates of intensive care unit (ICU) admission and hospitalization in a conventional ward (HC) in this cohort. A meta-analysis of studies assessing early HCQ in COVID-19 outpatients was conducted to test consistency with available literature.
Detailed methods are provided in the Supplementary data (see Supplementary Methods). Briefly, this retrospective cohort study, reported according to the STROBE guidelines, was conducted in the day hospital of the Institut Hospitalo-Universitaire (IHU) Méditerranéee Infection (https://www.mediterranee-infection.com/), Assistance Publique-Hôpitaux de Marseille (AP-HM), southern France, with an inclusion period from 17 March to 31 December 2020, and follow-up until 11 February 2021.
All patients with a positive PCR test regardless of symptoms were proposed to
come in our unit for treatment. We assessed the correlation between the Cycle
threshold value (Ct) of quantitative real-time PCR (qPCR) and viral viability in
culture for 3790 SARS-CoV-2 positive samples [16]. Using our culture protocol,
1941 were positive with an inverse correlation with the Ct, while culture
positivity rate was still 70% and 20% for Ct of 25 and 30, respectively. For Ct
of 35, only 3% of samples were positive in culture. In the present study, all
included patients had a qPCR Ct
Our center was the first to perform PCR screening since February, 2020 [17],
whereas mass screening was generalized in France since summer, 2020. Thus,
patients with a positive PCR or antigenic test performed outside our center
including in private laboratories could come for treatment at IHU but were
systematically confirmed by a rapid PCR test (time-to-result
Age, sex, date of day hospital attendance, and treatment were collected for all patients. The presence of symptoms, the time between the onset of symptoms and day hospital attendance and the time between the positive PCR sample and day hospital attendance were collected only for a subgroup of patients who were managed in December 2020. Compared to previous retrospective cohort studies of our center [15], this study focused on outpatients with ambulatory treatment, namely, patients who presented with non-severe COVID-19 who returned home and were not immediately hospitalized in a conventional ward.
Detailed COVID-19 management and therapeutic protocol are provided in supplementary data. Briefly, patients were systematically administered HCQ at 200 mg tid for 10 days, AZ at 500 mg on day 1 and then 250 mg for 4 days in the absence of contraindications. HCQ+AZ was prescribed as off-label medication. Anticoagulants were added after the death of a 60-year-old physician with no previous medical history who progressed despite HCQ+AZ and died of severe coagulopathy, disseminated and pulmonary thrombosis, and on the basis of the literature [19, 20]. Zinc was added to the treatment protocol following the publication of a clinical study reporting its beneficial effect in combination with HCQ [21], its beneficial effect in vitro, and the association between zinc deficiency and the lethality rate of COVID-19 [14]. Fig. 1 (Ref. [22, 23]) shows the chronology of therapeutic changes.
 Fig. 1.
Fig. 1.Pandemic periods, changes in therapeutic protocol and number of ambulatory patients treated in our daycare hospital (n = 10,429). HCQ, Hydroxychloroquine; AZ, Azithromycin. The three pandemic periods corresponded to different SARS-CoV-2 variants (Marseille-0 similar to the Wuhan strain for Act I, Marseille-1 for Act II, Marseille-4 and others for Act III—see [22, 23] and Supplementary data). Red crosses represent deaths.
The primary objective was to evaluate the age-specific 6-week IFR of unselected adult outpatients infected with SARS-CoV-2 who were managed early in a dedicated COVID-19 day hospital offering standardized treatment based on HCQ+AZ. The secondary objective was to test whether the use of HCQ+AZ was associated with improved IFR and lower ICU and HC rates in this cohort. The main considered confounding factors were age, sex, and epidemic period. The comprehensiveness of the HC cases, ICU transfers and deaths was optimized by using an automatic query of the informatic system of the APHM (Departement d’Information Médicale (DIM)) and, for deaths only, the National Register of Deceased Persons (NRDP) accessed on March 2021, which included reported deaths for 2020 and January and February 2021 [24]. In agreement, the deaths were collected for all patients regardless of the place of death (in hospital or not) in France.
Associations between treatment (HCQ+AZ), age, sex and epidemic period, and
clinical outcomes (deaths, ICU admissions, HC) were estimated using multivariable
logistic regression with adjustments for age, sex and epidemic period. A
two-sided
In 2020, 11,725 COVID-19 patients were treated and followed in our day hospital. Among these, 504 were immediately hospitalized in the conventional ward and were excluded. Among 11,221 outpatients, 792 were excluded for the following reasons: 424 patients with unavailable information on treatment, 265 minor patients, 82 considered cured, and 72 without a positive PCR test (though one patient could have been excluded for more than one reason) (Fig. 2). None refused the use of their data. After exclusion of these patients, our ambulatory cohort included 10,429 outpatients.
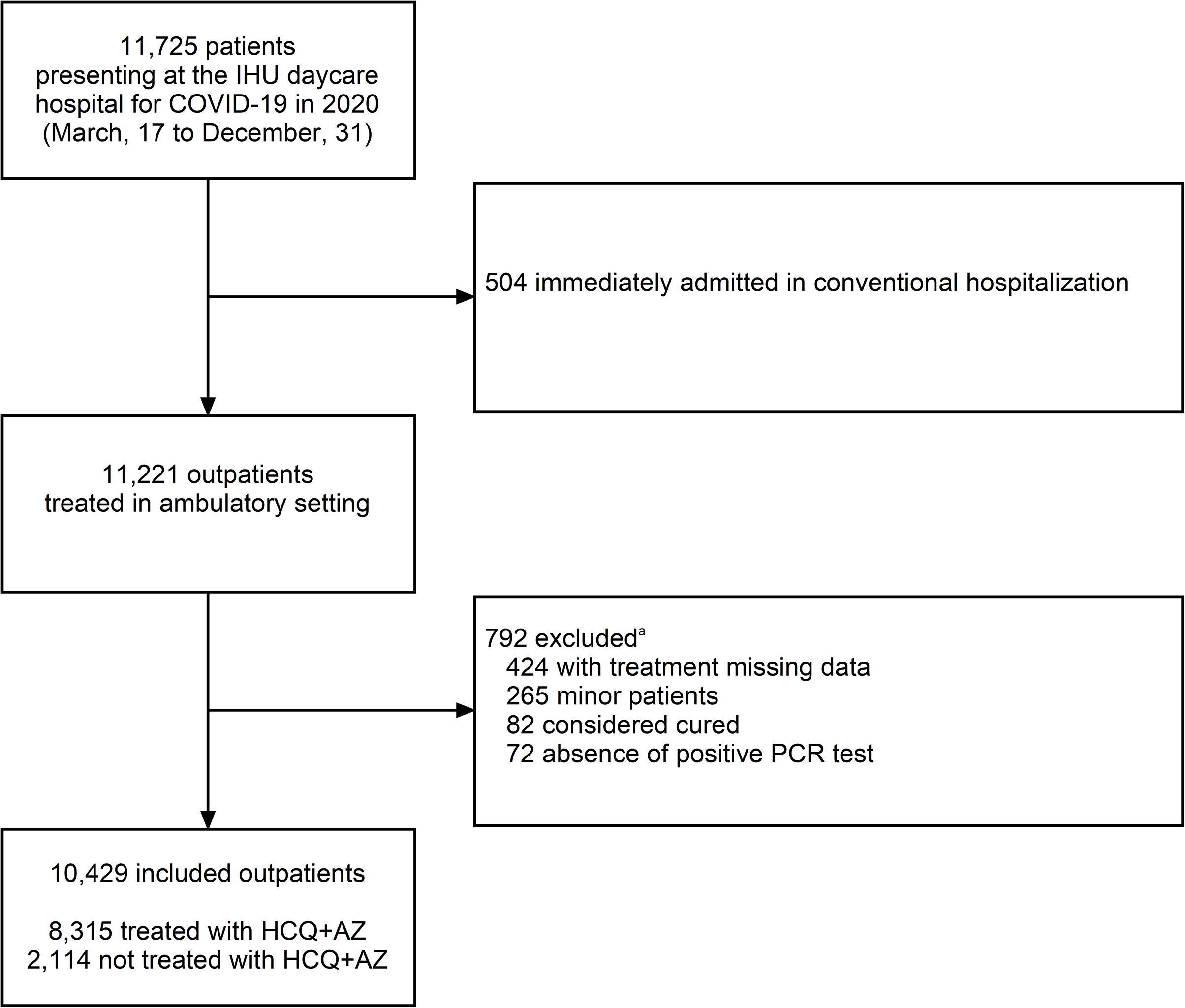 Fig. 2.
Fig. 2.Study flowchart.
The trend in the number of patients seen in the day hospital per week is shown in Fig. 1 and reflects 3 pandemic periods corresponding to different variants [22, 23]. The median age was 45 [IQR 32–57] years, and 5597 [53.7%] were women. Age and the sex ratio differed according to the epidemic period (Supplementary Table 1, Supplementary Fig. 1), with patients being older during the third period. The median delay from symptom onset to day hospital attendance was 4 days (interquartile range 2 to 6 days, information available for 1066 symptomatic patients seen in December 2020), and that from the screening positive test was 1 day (1–3 days, information available for 1119 patients). These delays were very similar among all age intervals (Supplementary Table 2). Among 1119 patients treated in December 2020, 53 (4.7%) were asymptomatic at presentation.
Among the 10,429 included patients, 8315 received the combination therapy HCQ+AZ (79.7%), 1091 received AZ alone (10.5%), 207 received HCQ alone (2.0%—mainly the first week, Fig. 1), and 816 did not receive either HCQ or AZ (7.8%). The reasons for not prescribing treatment are mentioned in Supplementary Table 3. No serious adverse events nor torsade de pointes was observed. Of these 10,429 patients, 21 had a second SARS-CoV-2 infection (0.2%) with a median time to reinfection of 160 days (interquartile range 127 to 209 days).
Among the 10,429 ambulatory patients, there were 16 deaths (0.15%) (Table 1,
Figs. 1,3. No patient under 60 years of age died (0/8414 (0%), 95% confidence
interval 0.0% to 0.4%) (Fig. 3). Therefore, the IFR among the 2015 patients
aged 60 and over was 0.8%. 11/16 deaths (70%) were common to both data sources
(DIM & NRDP). Two were identified only with the DIM, and three were identified
only with the NRDP. The median age of the decedents was 78 years (interquartile
age 69–82 years), and 12/16 (75%) were male. Thirteen (81%) had a Charlson
score
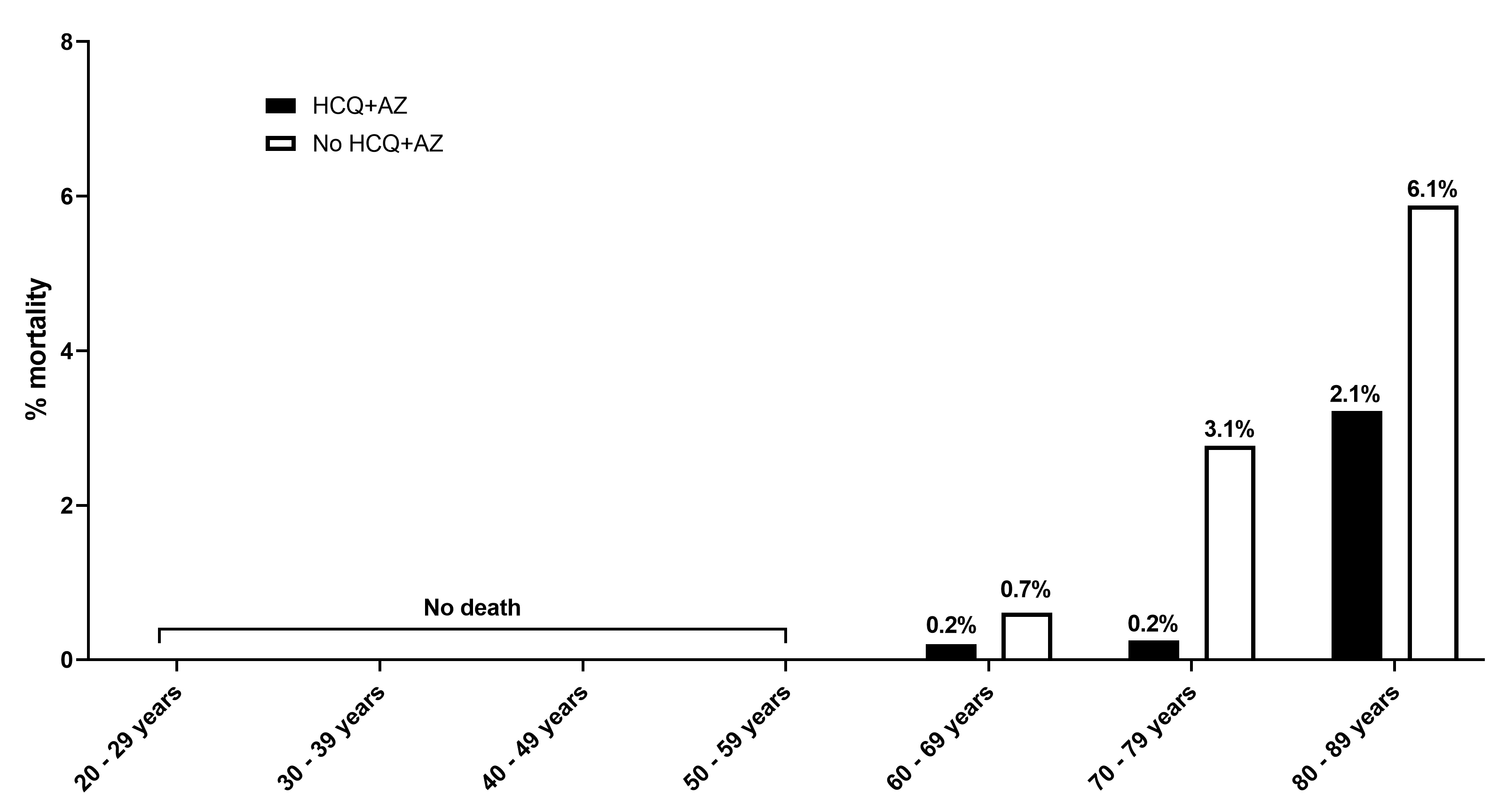 Fig. 3.
Fig. 3.Infection fatality rate by age class. HCQ+AZ,
hydroxychloroquine and azithromycin treatment. There were only 16 patients
| All | HCQ+AZ | Other treatments | |||||
| N | % | N | % | n | % | ||
| N | 16/10,429 |
0.15 | 5/8315 | 0.06 | 11/2114 | 0.52 | |
| Male sex | 4832/10,429 | 46.33 | 3914/8315 | 47.07 | 918/2114 | 43.42 | |
| Age interval (years) | |||||||
| 18–29 | 0/2157 | 0.00 | 0/1752 | 0.00 | 0/405 | 0.00 | |
| 30–39 | 0/2004 | 0.00 | 0/1650 | 0.00 | 0/354 | 0.00 | |
| 40–49 | 0/2074 | 0.00 | 0/1692 | 0.00 | 0/382 | 0.00 | |
| 50–59 | 0/2179 | 0.00 | 0/1726 | 0.00 | 0/453 | 0.00 | |
| 16/2015 | 0.79 | 5/1495 | 0.33 | 11/520 | 2.21 | ||
| 60–69 | 4/1286 | 0.31 | 2/1003 | 0.20 | 2/283 | 0.71 | |
| 70–79 | 6/555 | 1.08 | 1/395 | 0.25 | 5/160 | 3.13 | |
| 80–89 | 6/158 | 3.80 | 2/93 | 2.15 | 4/65 | 6.15 | |
| 0/16 | 0.00 | 0/4 | 0.00 | 0/12 | 0.00 | ||
| HCQ, Hydroxychloroquine; AZ, Azithromycin. | |||||||
There were 5 deaths among the 8315 patients who received HCQ+AZ (0.6 on 1000
patients) and 11 among the 2114 who received other treatments (p
| OR | 95% CI | p | ||
| Age (ref. 60–69 years) | ||||
| 70–79 | 2.81 | 0.88–8.96 | 0.0802 | |
| 8.29 | 2.52–27.20 | 0.0005 | ||
| Sex (ref. women) | ||||
| Men | 3.61 | 1.29–10.07 | 0.0145 | |
| Epidemic period (ref. period 1) | ||||
| Period 2 | 0.14 | 0.01–2.58 | 0.1856 | |
| Period 3 | 0.58 | 0.17–1.93 | 0.3743 | |
| Treatment (ref. no dual therapy) | ||||
| HCQ+AZ | 0.17 | 0.06–0.48 | 0.0007 | |
| OR, odds ratio; CI, confidence interval; Ref, reference; AZ, azithromycin; HCQ,
hydroxychloroquine. The two-way interaction between treatment and age was not statistically significant (p = 0.57). | ||||
Only 24 patients were transferred to the intensive care unit (0.23%), with no patient under 40 years of age being transferred (Supplementary Table 4). In the multivariable logistic regression, age and sex were associated with ICU transfer (Supplementary Table 5). Period 3 was associated with a nonsignificant (aOR 0.44, 0.19–1.02) 66% decrease in the risk of being transferred to the ICU independent of age, sex or HCQ+AZ treatment. HCQ+AZ was associated with a 44% nonsignificant (0.56, 0.24–1.30) decrease in the risk of ICU transfer (Supplementary Table 5).
Two hundred and seventy-eight patients (2.7%) were subsequently hospitalized (Supplementary Table 6). In the multivariable logistic regression, age, sex, and epidemic period, but not HCQ+AZ, were associated with the hospitalization rate. The hospitalization rate was decreased by 30–35% for periods 2 and 3 compared with period 1 (Supplementary Table 7).
Here, we demonstrated the feasibility and efficacy of early outpatient management with a combination HCQ+AZ treatment to prevent COVID-19-related death. In our cohort, as in the largest published ambulatory series (Table 3, Ref. [5, 6, 25, 26, 27, 28, 29, 30]), treatment with HCQ was not associated with serious cardiac side effects but was associated with a significant IFR decrease of 75%. The present cohort is among the largest cohorts of COVID-19 patients treated in the outpatient setting, with the lowest mortality rates: the IFR was 0.15% (0.06% among those treated with HCQ+AZ) versus 0.7% and 1.1% (0.30% and 0.39% among HCQ-treated patients) in the Iranian (169/22,784 patients with positive PCR) and Saudi ambulatory cohorts (61/5541 patients), respectively (Table 3) [5, 6].
In our cohort, the IFR among patients of all ages treated with HCQ+AZ was 60 per
100,000, which is much lower than the natural infection rate, even when evaluated
under the best conditions, as in Iceland, where it was estimated to be 300 per
100,000 [31]. The IFR was also estimated to be 89 per 100,000 in patients who
were
The cardiotoxicity of HCQ, previously considered irrelevant to oral administration and usual doses [33], has been exaggerated by studies with a potential conflict of interest, notably in the retracted article published in the Lancet [34]. White [33] showed that the concentrations needed to inhibit the hERG channel responsible for QT prolongation were 4 to 14 times higher than the concentrations observed in plasma at usual doses. In our center, we developed a smartwatch electrocardiogram and artificial intelligence for assessing the cardiac rhythm safety of HCQ+AZ and did not find any QTc prolongation [35]. In the literature, ambulatory and not critically ill patients with COVID-19 treated with hydroxychloroquine, azithromycin and/or antiretrovirals develop a significant, but not relevant, QT interval prolongation [36]. As shown in our cohort, a simple clinical and biological evaluation with blood potassium assessment and the use of a first electrocardiogram allowed us to initiate treatment with acceptable safety in terms of potential arrhythmias.
The Fig. 4 show, in a meta-analysis, that all the studies carried out on
outpatients, with minimal quality criteria (biological diagnosis, representative
population with adequate control and at least 1 death), are all in the same
direction (Table 3, Supplementary Tables 8,9, Supplementary Fig.
2). All these studies reported a similar magnitude (3-fold decrease in the risk
of death), and showed that early treatment with hydroxychloroquine improve
survival in COVID-19 outpatients in the community (n = 32,124 patients in 5
countries, Odds ratio 0.31 [0.20–0.47]) without heterogeneity (I
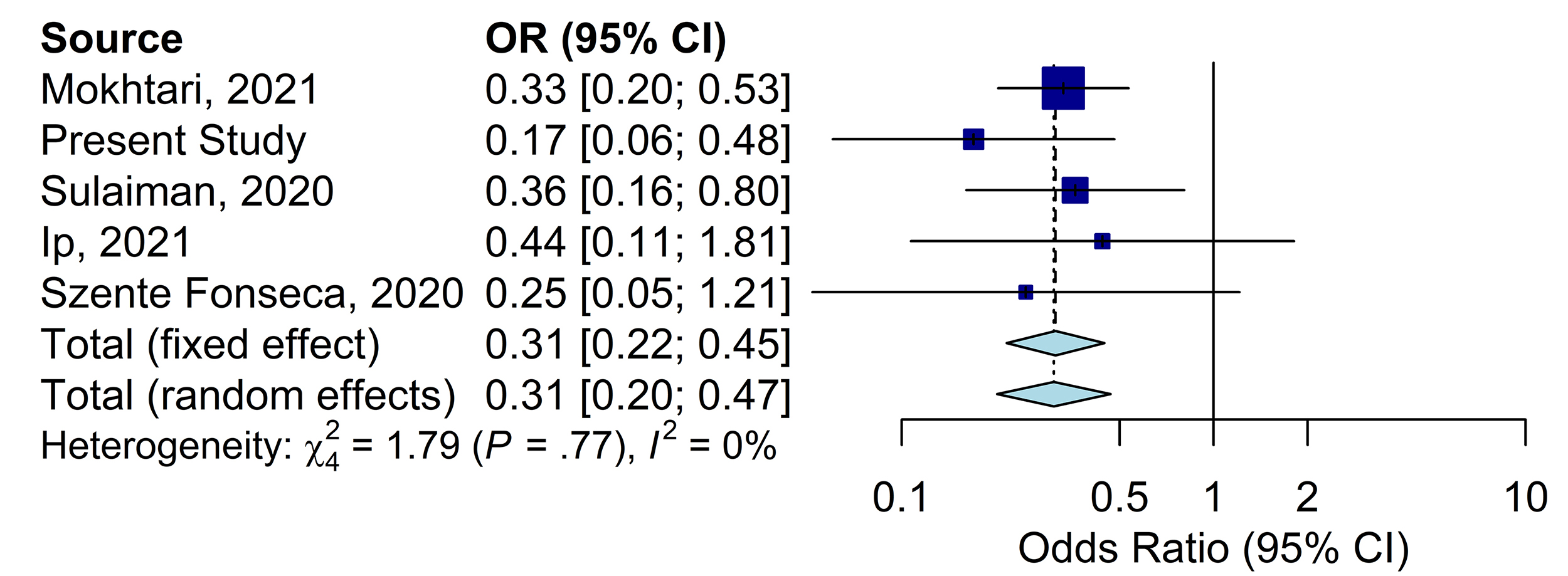 Fig. 4.
Fig. 4.Meta-analysis on studies in the community evaluating HCQ as an
ambulatory treatment to prevent COVID-19 mortality (n = 32,124). The
Meta-analysis was performed using random effects modeling for odds ratios (OR).
Chi square based Q test and I
| Study | Country | Treatment | Total sample size | Treated | Not treated | Overall mortality/1000 | Odds ratio (95% CI) | p-value |
| 95% CI | ||||||||
| Studies controlling for age | ||||||||
| Mokhtari et al., 2021 [5] | Iran | HCQ | 22,784 |
5964 |
16,820 |
7.0 | aOR 0.33 (0.21–0.55) | 0.0000082 |
| Present Study | France | HCQ+AZ | 10,429 | 1495 |
520 |
1.5 | aOR 0.17 (0.06–0.48) | 0.0007 |
| Sulaiman et al., 2020 [6] | Saudi Arabia | HCQ | 5541 | 1817 | 3724 | 11.0 | aOR 0.36 (0.16–0.80) | 0.012 |
| Ip et al., 2021 [25] | USA | HCQ | 1274 | 97 |
970 |
40.0 | aOR 0.44 (0.11–1.86) | 0.38 |
| Lima-Morales et al., 2021 [26] | Mexico | IVM+AZ+Montelukast+Acetylsalicylic acid | 768 | 481 | 287 | 87.2 | aOR 0.19 (0.10–0.36) | |
| Reis et al., 2021 (RCT) [27] | Brazil | HCQ | 606 | 198 | 408 | 5.0 | Only 3 deaths in untreated | 0.30 |
| Studies not controlling for age | ||||||||
| Szente Fonseca et al., 2020 [28] | Brazil | HCQ | 717 | 334 | 383 | 15.3 | Unadj. OR 0.25 (0.05–1.17) | 0.10 |
| Seftel et al., 2021 [29] | USA | Fluvoxamine | 113 | 65 | 48 | 8.8 | Only 1 death in untreated | 0.85 |
| HCQ, hydroxychloroquine; AZ, azithromycin; IVM, ivermectin; RCT; randomized
controlled trial; aOR, adjusted Odds ratio; 95% CI, 95% confidence interval. | ||||||||
The major limitation of the present cohort is its lack of assessment of comorbidities. Patients not receiving HCQ tend to be older and have more comorbidities [28], and although we were able to control for age and sex, the present study was not adjusted for other key comorbidities and may be biased accordingly. However, consistency with 3 similar studies assessing the death outcome and controlling for comorbidities [5, 6, 25] was demonstrated by meta-analysis. In another study by Szente-Fonseca et al. [28], a beneficial effect of hydroxychloroquine, alone or in combination with prednisone, was found for hospitalization and death, but adjusted model was provided only for the hospitalization outcome. Taken together, these results suggest that early treatment with HCQ was consistently reported to prevent progression of COVID-19 in ambulatory patients, independently of age, gender, and comorbidities.
All outpatients reported here were considered non-severe by the day-hospital physician based on routine assessment of saturation and dyspnea, but data on accurate initial clinical assessment was not collected. Follow-up was not systematically proposed after May 2020, so hospitalizations in and transfers to critical care units outside our city hospitals (APHM) may have been overlooked. However, deaths were identified through the French national register, thereby controlling this bias. The strengths of our study include the large sample size, the homogeneous management of patients associated with the monocentric design, and the double collection of death data by means of two registers: a local (city public hospital system) and national (French National Register of Deceased Persons) register.
The evolving concept and success of sequenced multidrug therapy including either HCQ, fluvoxamin or ivermectin for outpatient COVID-19 in reducing hospitalization and death by ~85% was reported by several authors without any link of interest with our team which strengths the external validity of the present study (Table 3, Fig. 4) [2, 3, 4, 42]. In another non-hospital context, the efficacy of HCQ in nursing homes (Supplementary Table 8) [43, 44, 45] strengthen the external validity and further highlight the role of HCQ in the non-hospital setting.
Finally, there are old and nontoxic drugs with in vitro and preliminary clinical efficacy on SARS-CoV-2 infection, such as HCQ, zinc, ivermectin, or fluvoxamine [2, 3, 4, 21, 39, 46]. Early oxygen, immunomodulation and antithrombotic therapy may also prevent disease worsening [3, 47]. Such drugs may be neglected when political factors, massive funding and fear lead to irrational decisions [48], such as therapeutic nihilism [2]. It seems urgent that governments and health authorities take in hand the evaluation of nonprofitable drugs, which are probably more effective than the drugs developed for this pandemic. This requires a profound paradigm shift, the extent of which was revealed by COVID-19 and which would be in line with the reflections on Tamiflu, recently documented in the British Medical Journal [48]. As long as the planned obsolescence of drugs continues and remains the current standard in Western countries, these richest countries and, theoretically, the most scientifically advanced, will remain those with the highest COVID-19 fatality rate in the world.
HCQ, hydroxychloroquine; AZ, azithromycin; IFR, infection fatality rate.
MMillion designed and supervised the study, conducted the investigation, formal analysis and wrote the first draft of the manuscript, JCL participated in the study design, investigation and critical review, all authors (HTD, IR, CD, CT, NC, SA, CA, KB, BD, SE, MH, MMailhe, CP, PS, CT, SG, EJ, AGG, HC, LCJ, PC, PG, PEF, BM, JCD, PH, JYG, AJ, SH, KGL, YO, PP, PB) participated in the investigation and provided constructive criticism and comments, LD, CB and SC conducted the formal analysis and meta-analysis, DR designed and supervised the study, and final proofreading with key finalization of the text.
Data presented herein were collected retrospectively from the routine care
setting using the electronic health recording system of the hospital. The
retrospective nature of the study was approved by our institutional review board
committee (Méditerranée Infection N
This manuscript has been edited by a native English speaker. We thank the reviewers who helped in substantially improving and clarifying the manuscript with their many comments and suggestions. We are thankful to Sylvie Arlotto, Marion Bechet, Yacine Belkhir, Pierre Dudouet, Véronique Filosa, Marie-Thérèse Jimeno, Alexandra Kotovtchikhine, Line Meddeb, Cléa Melenotte, Malika Mokhtari, and Pierre Pinzelli. All medical students from Aix Marseille University; all nurses; all laboratory staff; all administrative, technical and security staff from Assistance Publique-Hôpitaux de Marseille and IHU Méditerranée Infection; all volunteer medical doctors; and the Bataillon des Marins Pompiers de Marseille for their help. Other medical volunteers who contributed to taking care of COVID-19 patients in 2020 are acknowledged at the end of the Supplementary data.
This work was funded by ANR-15-CE36-0004-01 and by ANR “Investissements d’avenir”, Méditerranée infection 10-IAHU-03 and was also supported by Région Provence-Alpes-Côte d’Azur. This work received financial support from the Mediterranean Infection Foundation.
The authors declare no conflict of interest. The funders/sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. Funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Our group used widely available generic drugs distributed by many pharmaceutical companies.
