† These authors contributed equally.
Academic Editor: Peter Kokkinos
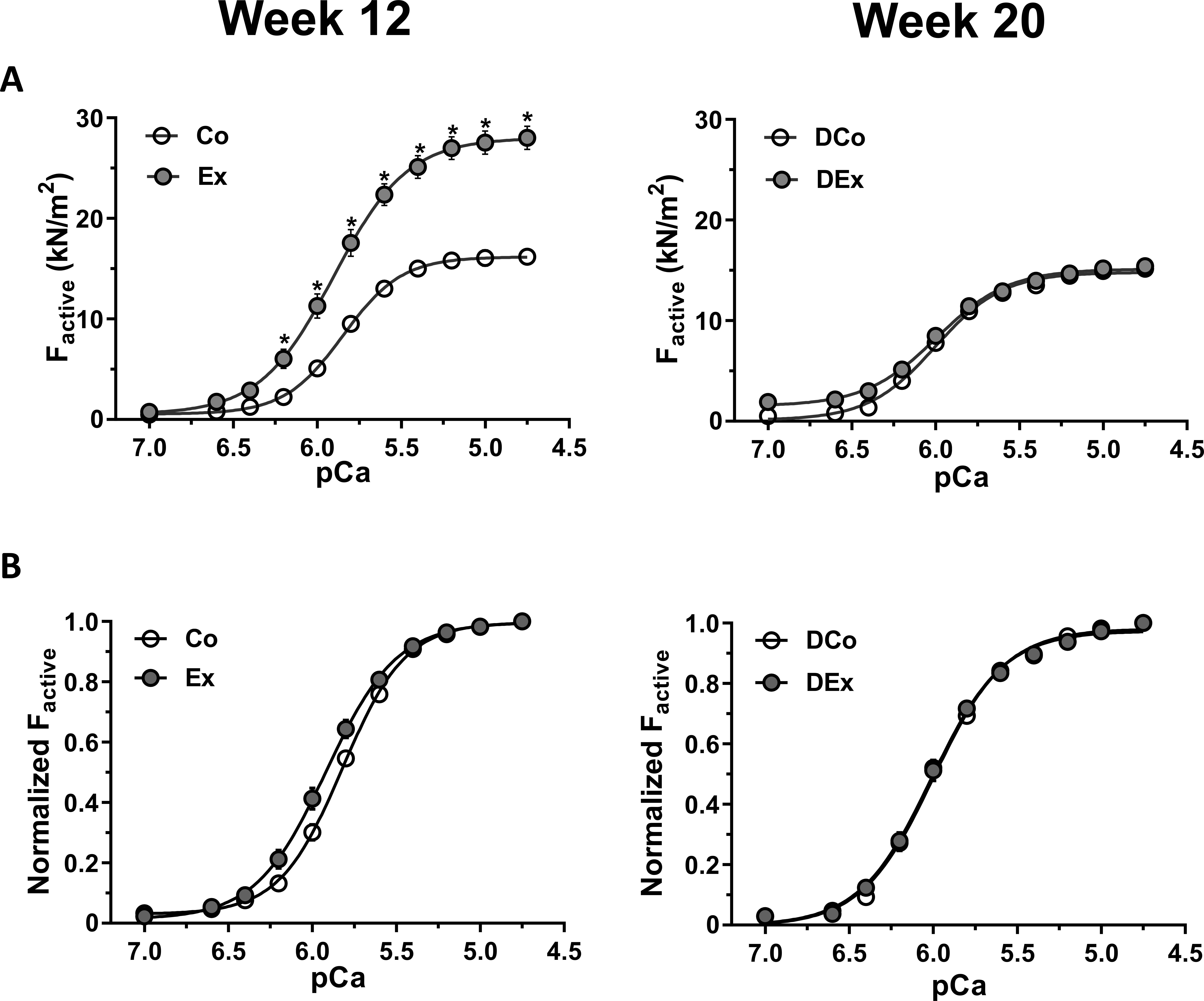
Although the knowledge of sports cardiology advanced significantly in the recent
years, the molecular mechanisms by which exercise training augments cardiac
performance is poorly understood. Here we aimed at determining left ventricular
(LV) myocardial sarcomeric protein modifications in a rat model of exercise
training and detraining. Young male Wistar rats were divided into exercised (Ex)
and control (Co) groups. Trained rats swam 200 min/day for 12 weeks. Detrained
(DEx) and control (DCo) rats remained sedentary for 8 weeks after completion of
the 12-week-long protocol. Ca