Academic Editors: Grigorios Korosoglou and Francesco Nappi
Transthoracic echocardiography (TTE) and Cardiac Magnetic Resonance (CMR) have complementary roles in the severity grading of mitral regurgitation (MR). Our objective was to systematically review the correlation of MR severity as assessed by TTE and CMR. We searched MEDLINE and Cochrane Library for original series published between January 1st, 2000 and March 23rd, 2020. We used Cohen’s kappa coefficient to measure agreement between modalities. We plotted a hierarchical summary receiver operator characteristic (HSROC) curve and estimated the area under the curve (AUC) to assess the concordance between the two imaging modalities for the detection of severe MR. We identified 858 studies, of which 65 underwent full-text assessment and 8 were included in the meta-analysis. A total of 718 patients were included (425 males, 59%) in the final analysis. There was significant heterogeneity in the methods used and considerable variation in kappa coefficient, ranging from 0.10 to 0.48. Seven out of eight studies provided the necessary data to plot HSROC curves and calculate the AUC. The AUC for detecting severe MR was 0.83 (95% CI 0.80 to 0.86), whereas the AUC for detecting moderate to severe MR was 0.83 (95% CI 0.79 to 0.86). The agreement between TTE and CMR in MR severity evaluation is modest across the entire spectrum of severity grading. However, when focusing on patients with at least moderate MR the concordance between TTE and CMR is very good. Further prospective studies comparing hard clinical endpoints based on the CMR and TTE assessment of MR severity are needed.
Mitral regurgitation (MR) is the second most common valvular disorder requiring surgical or transcatheter intervention [1, 2, 3, 4]. Myxomatous degeneration of the valve leaflets is the main cause of primary MR, whereas secondary MR most commonly occurs in cardiomyopathies (ischemic or dilated) that result in tethering of valve leaflets or annular dilatation [4, 5, 6]. Chronic MR leads to volume overload, left ventricular (LV) dilatation, and eventually LV systolic dysfunction and heart failure [7, 8].
Transthoracic echocardiography (TTE) is the imaging modality of choice for the diagnosis, quantification, and classification of MR according to the ACC/AHA guidelines [9, 10]. A multiparametric approach including qualitative (e.g., dense, triangular signal on continuous wave Doppler), semi-quantitative (e.g., vena contracta width) and quantitative indices (e.g., effective regurgitant orifice area) is recommended for the complete evaluation of MR severity [11, 12]. Although TTE is broadly available and cost-effective, poor acoustic windows or eccentric jets may limit its use [13, 14, 15]. CMR can be used in such difficult cases as a complementary tool for the assessment and quantification of MR given its high diagnostic accuracy and reproducibility in the assessment of ventricular volumes [1, 16, 17, 18, 19, 20]. Limitations of CMR include the limited availability of the technique and also that discrepancy in measurements and results is possible. First, variability can arise in LV volumes quantification due to errors in basal LV slice determination and endocardium delineation (i.e., inclusion of papillary muscles). Second, velocity mapping can lead to altered results depending on the plane chosen to measure aortic flow [21]. Arrhythmias such as atrial fibrillation or premature ventricular contractions can pose challenges in images acquisition because images are acquired from consecutive cardiac cycles using electrocardiogram gating. Those sources of heterogeneity can lead to varying degrees of agreement between the two modalities in MR evaluation [22, 23, 24].
 Fig. 1.
Fig. 1.PRISMA flow diagram. The exact number of articles included or excluded in each step of the process according to Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.
We performed a systematic review and meta-analysis of the agreement of CMR and two-dimensional-(2D) TTE to evaluate MR severity.
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [25].
MEDLINE and Cochrane Library were searched for relevant articles using the following search algorithm: ((“mitral regurgitation”) OR (“mitral insufficiency”)) AND ((“magnetic resonance imaging”) OR (“cardiovascular magnetic resonance”) OR (“CMR”) OR (“MRI”) OR (“cardiac magnetic resonance”)).
After duplicate removal, titles and abstracts were independently screened by two reviewers (IB and AE) in order to identify studies fulfilling the following inclusion criteria: (1) studies comparing MR severity between 2D-TTE and CMR, irrespective of parameters assessed and cut-off values used, (2) studies reporting Cohen’s kappa coefficient or provided sufficient data to calculate it, (3) published in any language up to March 2020. Two authors (CAP and DGK) independently assessed the eligibility of the potentially included studies.
A pre-specified form was used to extract the following demographics and baseline characteristics of the included studies: author’s name, year of publication, country, study design, time between TTE and CMR performance in days, number of patients, basic characteristics of participants (age, sex, MR etiology), TTE and CMR parameters used to assess MR severity and their cut-offs, kappa coefficient and its 95% confidence interval (CI), and the number and severity of MR cases identified by each imaging modality.
The primary objective was to assess the correlation of MR severity grading between CMR and 2D-TTE. The secondary objective was to evaluate the degree of concordance between 2D-TTE and CMR in detecting firstly severe MR and secondly moderate to severe or severe MR.
Wherever the kappa coefficient was not reported, we calculated it using relevant formulas along with its standard error (SE) and 95% CI. A value of kappa lower than 0.20 indicates poor agreement, while k = 0.21–0.40 a fair, k = 0.41–0.60 a moderate, k = 0.61–0.80 a substantial, and k = 0.81–1.00 an almost perfect agreement [26].
Given the considerable heterogeneity in the parameters used for MR assessment across the included studies, the hierarchical summary receiver operator characteristic (HSROC) curve and the corresponding area under the curve was used to determine the level of concordance between 2D-TTE and CMR in detecting severe or moderate to severe MR [27].
Revman version 5.3 (Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014) and Stata 13.0 (StataCorp, College Station, Texas, USA) was used for all analyses.
In total 858 studies were identified through the literature search. After screening of titles and abstracts 65 studies were eligible for full-text assessment. Finally, eight studies, including 718 patients, fulfilled the inclusion criteria and were incorporated into the final analysis. The detailed search strategy is illustrated in Fig. 1.
The number of enrolled patients ranged from 33 to 258 among the included
studies. The mean age of the total population was 58.1
| First Author | Publication year | Country | Study design | Patients | Age mean, years (SD) | Male (%) | Type of MR |
| Gelfand et al. [32] | 2006 | USA | retrospective | 83 | 55 (14.9) | 47 (56.6%) | Not mentioned |
| Heitner et al. [29] | 2012 | USA | retrospective | 68 | 62 (10) | 41 (60.3%) | Mixed |
| Uretsky et al. [22] | 2015 | USA | prospective | 103 | 61 (14) | 59 (57.2%) | Primary: degenerative (47%) |
| Lopez-Mattei et al. [23] | 2016 | USA | retrospective | 70 | 61 (10) | 36 (51.4%) | Primary (50%) |
| Secondary (50%) | |||||||
| Penicka et al. [24] | 2018 | Belgium/Czech Republic | prospective | 258 | 63 (14) | 155 (60%) | Primary (100%): flail (25%), prolapse (75%) |
| Jang et al. [30] | 2018 | Korea | prospective | 33 | 52 (9) | 27 (81.8%) | Primary (100%): prolapse or flail |
| Levy et al. [31] | 2018 | France/Monaco | prospective | 53 | 64 (12) | 37 (70%) | Primary (100%) |
| Hassan et al. [28] | 2020 | Egypt | prospective | 50 | 47 (16.8) | 23 (46%) | Primary (60%) |
| Secondary (40%) |
| First author | Time of imaging between TTE – CMR median, days | TTE parameters | CMR parameters | CMR method for MR quantification | Cohen’s kappa coefficient (95% CI) | Grading categories |
| Gelfand et al. [32] | 31 | Jet area/LA area, jet’s turbulence –eccentricity, pulmonary vein flow | RF: mild |
LVSV-AoPC | 0.39 (0.25, 0.52) | Mild, moderate, moderate to severe, severe |
| Hassan et al. [28] | 1 | ASE multiparametric approach [35] | RV: mild |
LVSV-AoPC | 0.19 | Mild (CMR only), moderate, severe |
| Heitner et al. [29] | 2 | Integrated approach (Vena contracta, pulmonary vein flow, jet area, LA size, LVESD) | Integrated approach (Vena contracta, jet intensity, jet area, jet length, LA size, LVESD) | Four criteria; vena contracta size, jet intensity, jet area and length | 0.47 (0.29, 0.65) | Insignificant or mild, moderate or severe |
| Jang et al. [30] | 1 | ASE multiparametric approach [35], RV |
RV: mild |
LVSV-AoPC | 0.10 (–0.05, 0.24) |
Mild, moderate, severe |
| Levy et al. [31] | 1 | PISA RV: mild |
RV: mild |
LVSV-AoPC | 0.32 (0.14, 0.51) | Mild, mild to moderate, moderate to severe, severe |
| Lopez-Mattei et al. [23] | 3 | ASE multiparametric approach [35] | ASE multiparametric approach [35] | LVSV-AoPC | 0.44 (0.34, 0.54) | Mild, moderate, moderate to severe, severe |
| Penicka et al. [24] | 1 | ASE multiparametric approach [34] | RV: severe |
LVSV-AoPC | 0.48 (0.38, 0.59) | Moderate, severe |
| Uretsky et al. [22] | 15 | ASE multiparametric approach [35] | RV: mild |
LVSV-AoPC | 0.14 (0.04, 0.24) | Mild, moderate, severe |
| ACC/AHA, American College of Cardiology/American Heart Association; AoPC, Aortic
Phase-Contrast forward volume; ASE, American Society of Echocardiography; CI,
Confidence Interval; CMR, Cardiovascular Magnetic Resonance; LA, Left Atrium;
LVESD, Left Ventricular End-Systolic Diameter; LVSV, Left Ventricular Stroke
Volume; MR, Mitral Regurgitation; PISA, Proximal Isovelocity Surface Area; RF,
Regurgitant Fraction; RV, Regurgitant Volume; TTE, Transthoracic
Echocardiography. RVPISA, RV calculated with PISA method. RVAC, Angle Correction (AC) method of RVPISA. | ||||||
Cohen’s kappa coefficient varied significantly among the included studies (ranging from 0.10 to 0.48). Heitner et al. [29] and Penicka et al. [24] found moderate agreement between TTE and CMR; [k = 0.47 (95% CI 0.29 to 0.65) and k = 0.48 (95% CI 0.38 to 0.58), respectively]. On the other hand, Jang et al. [30] and Uretsky et al. [22] reported poor agreement between the two imaging modalities [k = 0.10 (95% CI –0.04 to 0.24) and k = 0.14 (95% CI 0.04 to 0.24), respectively].
Seven out of eight studies provided the necessary data to plot HSROC curves and calculate the AUC. The AUC for detecting severe MR was 0.83 (95% CI 0.80 to 0.86) (Fig. 2) and 0.83 (95% CI 0.79 to 0.86) for detecting moderate to severe MR (Fig. 3).
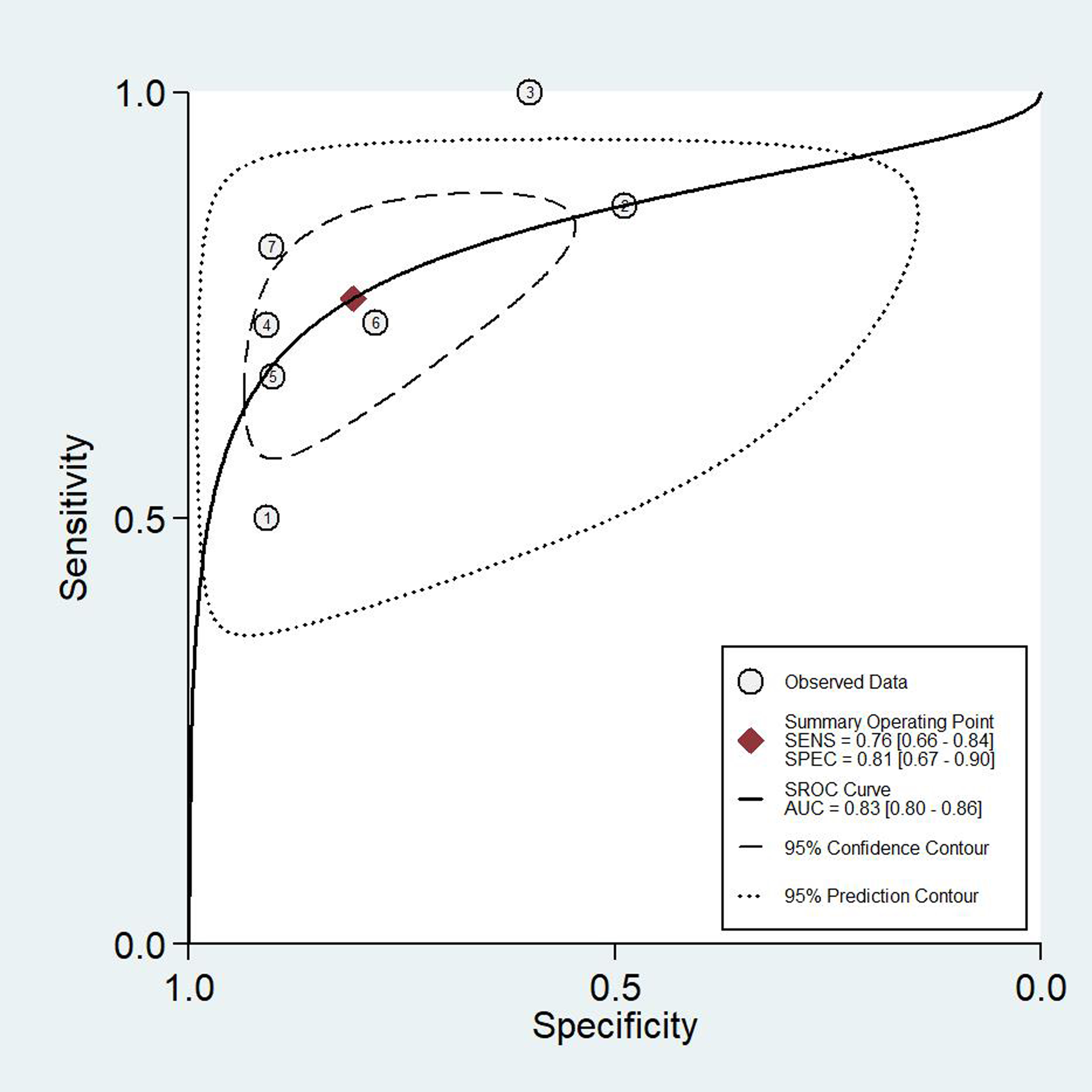 Fig. 2.
Fig. 2.HSROC curve and the AUC for detecting severe MR. The HSROC curve demonstrating the accuracy of TTE in diagnosing severe MR with CMR as reference standard. CMR, Cardiac Magnetic Resonance; HSROC, Hierarchical Summary Receiver Operator Characteristic; MR, Mitral Regurgitation; TTE, Transthoracic Echocardiography.
 Fig. 3.
Fig. 3.HSROC curve and the AUC for detecting moderate to severe and/or severe MR. The HSROC curve demonstrating the accuracy of TTE in diagnosing moderate to severe and/or severe MR with CMR as reference standard. CMR, Cardiac Magnetic Resonance; HSROC, Hierarchical Summary Receiver Operator Characteristic; MR, Mitral Regurgitation; TTE, Transthoracic Echocardiography.
This systematic review and meta-analysis assessed the correlation of MR severity by 2D-TTE and CMR showed that the agreement between the two modalities ranges from poor to moderate across the entire spectrum of severity grading. However, when focusing on the group of patients with advanced disease, i.e., moderate- to- severe or severe MR, the two imaging modalities show good agreement.
A previous study by Sköldborg et al. [37] used regurgitant volume as the only echocardiographic criterion of MR severity, comparing it with CMR-calculated regurgitant volume. However, to our knowledge, our study is the first systematic review including studies where the regurgitation was assessed in an integrative echocardiographic approach based on a multitude of parameters as currently recommended by international guidelines [10, 33] given that no single echocardiographic parameter has high enough accuracy and reproducibility to constitute the sole criterion for the diagnosis of severe MR [38, 39]. Furthermore, a quantitative echo approach is often not feasible in practice. In a recent study, regurgitant volume calculation was possible in only 44 out of 72 patients [40].
TTE is the guideline-directed first-line imaging test for the diagnosis and management of MR. A multiparametric approach that combines several TTE indices is sufficient for evaluating MR in most patients [10, 36]. In cases of poor image quality or discrepancy between clinical and TTE findings, ACC/AHA guidelines recommend either transesophageal echocardiography (TEE) or CMR for the accurate assessment of MR [33]. However, discordance in MR evaluation between CMR and 2D-TTE has been reported [18, 22, 34]. Herein, we confirm the presence of discordance between the two modalities and additionally report significant variation in MR grading. There are several reasons that could explain these findings. First, there was significant heterogeneity in the type of MR among the included studies. Secondary MR imposes a greater challenge for accurate echocardiographic evaluation [41]. Even the European and American guidelines disagree and have different cut-offs for defining severe secondary MR on TTE by the PISA method (regurgitant volume of 30 vs 60 mL/beat, respectively) while there is no specific consideration for the CMR cut-off [10, 33]. Second, the variability in the method used to calculate the regurgitant volume in either CMR or TTE could be a significant source of discrepancy [42]. Either the flow convergence method or the time-consuming and more prone to errors Doppler volumetric method [43, 44, 45], could affect the calculated regurgitant volume value significantly using echocardiography. The same applies to CMR, when determining the last basal slice at the mitral annulus in the planimetry-derived stroke volume calculation, especially when there is a marked descent of the base and the analysis software does not use long-axis planes for cross-referencing [46].
The PISA technique is limited in the following scenarios: (1) cannot be used in the presence of multiple jets, and (2) typically measured at a single-timepoint which could over or underrepresent the measurement across the length of systole, (3) becomes less accurate as the shape of the isovelocity shell deviates from hemispherical or is eccentric/wall-bound [47]. The MR jet is commonly elliptical and evaluation by vena contracta or proximal isovelocity surface area may underestimate MR severity [48]. Of note, quantitative methods are not routinely performed for mild MR. In addition, these 2D methods are dependent on significant geometrical assumptions in the measurement of the MR radius for the PISA equation and in evaluating the vena contracta, which are both not spherical in shape. Three-dimensional echocardiography allows for a more accurate assessment of MR severity with a high level of agreement with that obtained by standard CMR methods [47, 49]; however, such studies were not included in our analysis, considering that they are referring to transesophageal echocardiography. Another disadvantage of CMR is that image quality may be affected by irregular heart rhythms such as atrial fibrillation which is relatively common in this group of patients.
CMR allows for accurate LV volumetric assessment with excellent reproducibility,
which can be used for quantitative MR evaluation [1, 32, 50, 51, 52, 53].
Additionally, there is a relatively small but growing body of literature
regarding the association of valvular regurgitation evaluation by CMR with
outcomes in different clinical settings [22, 24, 54, 55, 56]. Using CMR-derived
regurgitant volume as a criterion, Myerson et al. [55] were able to
discriminate accurately the patients that would develop an indication for MR
surgery (RV
The current meta-analysis suggests that patients with significant MR on CMR can be accurately identified by both TTE and CMR. However, moderate MR on CMR is usually overestimated by TTE. This finding, combined with the prior works by Myerson and Penicka, suggests that a lower regurgitant volume cutoff may be needed for CMR as compared to echocardiography. If the cut-off for CMR-based regurgitant volume was lower, the agreement between TEE and CMR would have been better. The cut-off of 60 mL was extrapolated from TTE to CMR. Nonetheless, when different techniques are used to define severity in other valvular diseases such as aortic stenosis, another cut-off for aortic valve area is applied to define severe stenosis [57, 58, 59]. Therefore, further studies correlating the MR severity through different modalities and patients’ outcomes should be conducted.
Despite the moderate, at most, agreement between 2D-TTE and CMR evaluation of MR severity across the entire spectrum of severity grading, our study shows that the two imaging modalities are highly correlated when focusing on severe MR. This is in agreement with the established role of 2D-TTE in the clinical management of MR. Indeed, current ESC and ACC/AHA guidelines suggest surgical or transcatheter mitral valve intervention for symptomatic patients with severe MR or asymptomatic patients with severe MR and signs of LV decompensation or pulmonary hypertension using established 2D-echocardiographic methods for evaluating all these parameters: MR type and grade, LV size and function and estimating the pulmonary artery pressures [10, 33]. Furthermore, TTE is the modality of choice for follow-up of asymptomatic MR, evaluation of LV function with the volumetric method of ejection fraction and assessment of LV myocardial intrinsic dysfunction by evaluating the global longitudinal strain by speckle tracking for both primary and secondary MR providing guidance regarding the timing of intervention [60, 61]. The use of 3D-TEE can further address the challenges imposed on 2D-TTE by complex jets and geometrical assumptions, allowing for accurate assessment and grading of MR, by providing direct visualization of MV leaflets and other cardiac structures, such as pulmonary veins [43]. On the other hand, time-resolved 3D (or else 4D) flow phase-contrast CMR with a demonstrated reliability in intracardiac flow visualization might prove to be the answer to some of the limitations of conventional CMR techniques [62].
We performed a systematic review of the literature and statistical analysis that included HSROC curves to further delineate the correlation between 2D-TTE and CMR evaluation of MR severity. Our study has a number of limitations. First, the studies that we meta-analyzed were observational and included patients with different MR etiology. Secondly, MR was evaluated using a variety of echocardiographic and CMR parameters. Therefore, because of the heterogeneity in the parameters used for MR assessment, summary sensitivity, and specificity points could not be calculated. Moreover, MR grade is affected by LV afterload, which is defined by the peripheral blood pressure unless there is aortic valve stenosis. Thus, the time difference between the two exams CMR and TTE for MR evaluation may have had an impact on the MR grading.
In this systematic review and meta-analysis, we found a modest agreement between 2D-TTE and CMR for the evaluation of MR severity across the entire spectrum of severity grading. However, when focusing on patients with severe or moderate to severe MR, CMR and 2D-TTE showed good correlation. Large prospective clinical outcome studies are needed to provide further insights into the additive role of CMR in patients with MR.
ACC, American College of Cardiology; AHA, American Heart Association; AUC, Area Under the Curve; CI, Confidence Interval; CMR, Cardiac Magnetic Resonance; ESC, European Society of Cardiology; HSROC, Hierarchical Summary Receiver Operator Characteristic; LV, Left Ventricle; MR, Mitral Regurgitation; PISA, Proximal Isovelocity Area; RV, Regurgitant Volume; TEE, Transesophageal Echocardiography; TTE, Transthoracic Echocardiography.
CAP and TDK conceived the study; CAP, IB, AE, DGK and TDK designed the study while IB and AE did the electronic search and IB and TZ extracted the data; CP and DGK performed the statistical analysis. The final paper was written by IB, AE, CAP, DGK, TZ, GE, VK, OKK, PNK, TDK and then critically appraised and finally reviewed by all the authors.
Not applicable.
We would like to express our gratitude to peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest. Theodoros D. Karamitsos is serving as one of the Editorial Board members of this journal. We declare that Theodoros D. Karamitsos had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Francesco Nappi and Grigorios Korosoglou.
