† These authors contributed equally.
Academic Editors: Calogera Pisano and Luca Zanoli
Acute ischemic stroke (AIS) continues to be one of the most important medical and social problems in our country. Carotid endarterectomy (CEA) is the standard and effective surgical treatment for AIS prevention in patients with significant carotid artery stenosis. Even though CEA is a safe procedure when performed by an experienced surgeon, it is still associated with risks of operative complications inherent to any surgical intervention. Therefore, immediate postoperative appropriate adjuvant or neurological salvage therapy for AIS patients after CEA is necessary. In this study,we report three patients in our institution who received immediate post-operative interventional therapy for neurological salvage, in the setting of cerebral embolism after CEA.
Carotid endarterectomy (CEA) is an operation aimed at removing an atherosclerotic plaque located on the inner wall of the carotid artery. The operation is the restoration of the disturbed blood flow in the carotid artery [1]. CEA is an operation aimed at reducing the risk of acute ischemic stroke (AIS) and associated death [1]. However, AIS is also the most common complications. Perioperative AIS after CEA can be caused by the following reasons: embolism with a fragment of a plaque or thrombus, decompensation of cerebral blood flow against the background of clamping of the carotid artery, acute thrombosis or occlusion of arteries after surgery, dysfunction of a temporary intraluminal shunt [2]. The incidence of such complications currently ranges from 0.3 to 2.1%. About 80% of AIS patients happen within 24 hours after surgery [2, 3]. In the absence of technical equipment for performing CEA, treatment is typically conservative, and the potential for complete neurologic recovery is not good. Therefore, neurointerventional (endovascular) therapy may be an appropriate choice for AIS patients. For example, digital subtraction angiography (DSA) and endovascular mechanical thrombectomy [4], these measures may provide a new pathway for improving the prognosis of patients. We recently performed CEA on four patients who awakened with a profound neurologic deficit believed to be a result of intraoperative thromboembolism. These patients were successfully treated with interventional therapy for neurological salvage.
A 48-year-old patient had dyskinesia in his right upper limb for 9 days, so he sought treatment at a local hospital. Ultrasound of the carotid arteries showed that the left internal carotid artery (ICA) was stenosis (50–69%). Physical examination: satisfactory condition, clear consciousness, clear speech, absence of aphasia, sensitive light reflex, absence of central facial and language paralysis, no neurological deficit. The abdomen is soft, painless on palpation, the legs are free of swelling, muscle strength and sensitivity are normal.
Under general anesthesia, the left common carotid artery (CCA), ICA and external carotid artery (ECA), superior thyroid artery (STA) were exposed. After detecting a blockage of the carotid artery, its anterior wall is dissected. The intima of the internal carotid artery was carefully treated to remove the remaining plaque. Suture the carotid artery with a continuous suture, layer-by-layer suturing of the wound and install a drainage system in the projection of the carotid artery. After the operation, the patient did not wake up. Blood pressure (BP) 160/90 mmHg. Both pupils are round, slow to respond to light, the neck is soft; there are no signs of pathology from the heart and lungs. Poor activity of the left limb, the level of muscle strength is 0, the pathological reflex is positive.
After the patient was satisfied with local anesthesia, the right femoral artery
(RFA) was punctured according to the Seldinger technique, and the 6F guide tube
was placed in the petrosis segment of the right ICA. DSA
(Philips Medical Systems Nederland B.V.,
Eindhoven, Netherlands) showed the distal end of the upper trunk of the middle
cerebral artery (MCA) (Fig. 1). After superselective angiography (Philips Medical
Systems Nederland B.V., Eindhoven, Netherlands) confirmed that the distal artery
free, the solitaire stent 4
 Fig. 1.
Fig. 1.Images of Case 1. (A) Preoperative right common carotid artery
(CCA) arteriogram revealed a severe proximal internal carotid artery (ICA)
stenosis. (B) Preoperative right middle cerebral artery (CMA) and anterior
cerebral artery (ACA) arteriogram revealed normal. (C) The endometrium of the CCA
was dissected longitudinally and presented as muddy unstable plaques. (D)
Completed removal of plaque. (E) Incision suture. (F) Close the carotid sheath.
(G) Intimal plaque. (H) Postoperative right CCA arteriogram revealed the M1
segment of the right MCA is occluded. (I,J) Superselective angiography of a
microcatheter through an occluded segment reveals a distal image of the occluded
vessel. (K,L) Solitaire 4
The patient had no neurological deficit, round pupils on both sides, sensitive reflex, muscle strength of left upper limb was about 3 degrees, muscle strength of left lower limb was 4 degrees, right muscle tension was normal, and no pathological reflex was found.
A 48-year-old man had clearly impaired movement of his right upper limb 9 days ago. Color Doppler imaging (CDI, GE Medical System, Chicago, IL, USA) of the left ICA showed stenosis (50–69%). Physical examination: the right upper limb was paralyzed for 9 days. No other obvious positive signs. Hypertension for 2 years, oral antihypertensive drugs for 2 years, diabetes mellitus (DM) for 2 years, coronary heart disease (CHD), no hepatitis and tuberculosis, no history of drug allergy.
Under general anesthesia, the left CCA, ICA and ECA, STA were exposed. After the blockage of the artery, the anterior wall of the carotid artery is dissected. The plaque was presented as cloudy unstable, the intima of the ICA was carefully treated and fragments of the plaque were removed. Suturing the carotid artery with a continuous suture. Layer-by-layer suturing of the wound. The right lower limb was inhibited, and the femoral membrane was well fixed. The tension in the muscles of the limbs was normal. After the operation. The patient underwent a Computed Tomography (CT, Siemens, Forchheim, Germany) scan of the head, which ruled out intracerebral hemorrhage (ICH). DSA (Philips Medical Systems Nederland B.V., Eindhoven, Netherlands) has demonstrated that embolus forms during CEA (Fig. 2). Then, through the 6-French introducer sheath in the RFA and after the introduction of heparin (3000 U), the 6-French guide catheter (Cordis Corporation, Bridgewater, NJ, USA) was advanced into the proximal left CCA; the thrombotic plaque disappeared after the introduction of 0.5 mg of tirofiban (Grandpharma, Wuhan, China) into the artery. Postoperative arteriogram of the left ICA was found to be normal.
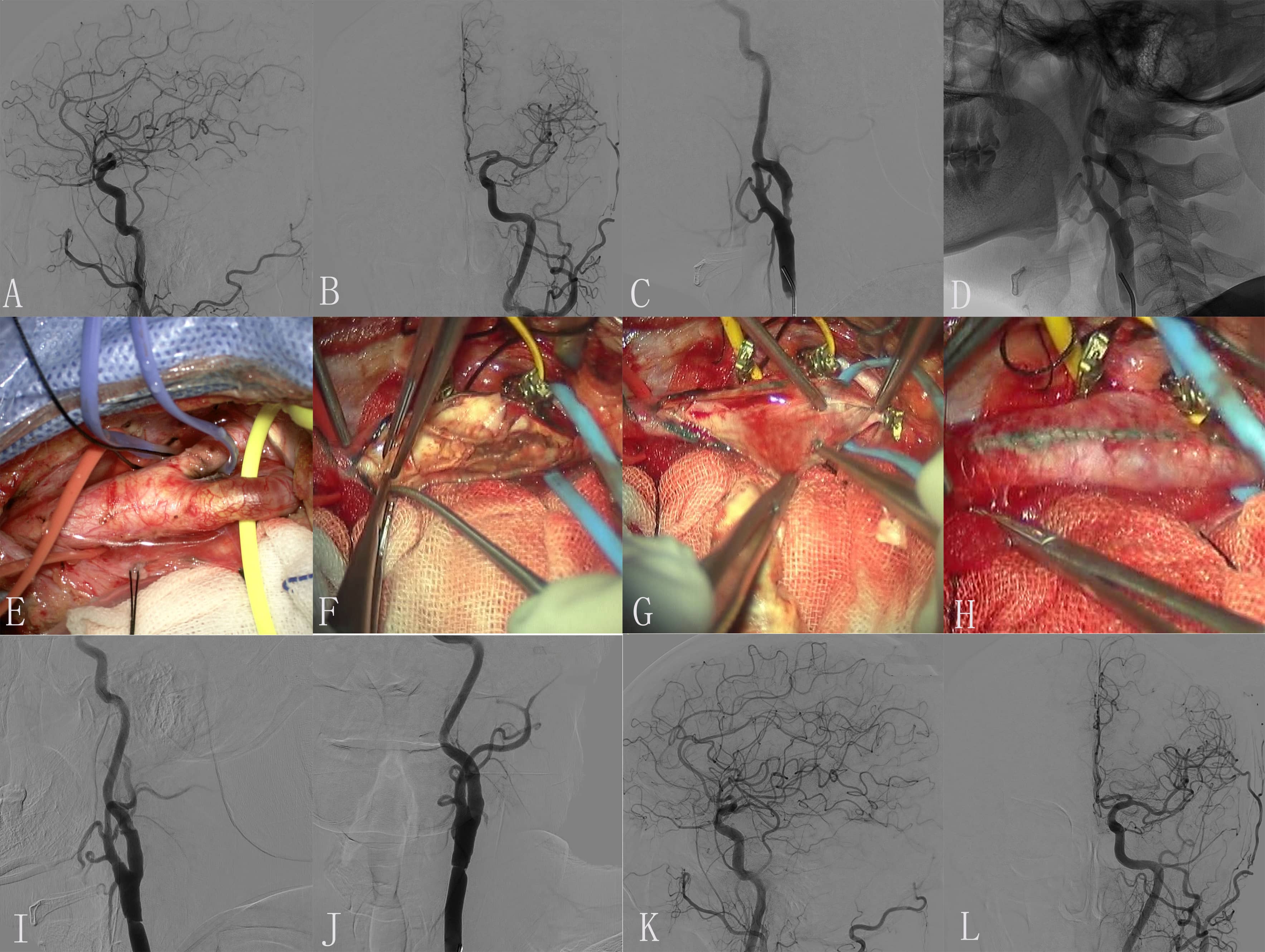 Fig. 2.
Fig. 2.Images of Case 2. (A,B) Preoperative left middle cerebral artery (MCA) and anterior cerebral artery (ACA) arteriogram revealed normal. (C,D) Preoperative left common carotid artery (CAA) arteriogram revealed a severe proximal internal carotid artery (ICA) stenosis. (E) Exposure of diseased vessel. (F) The endometrium of the CCA was dissected longitudinally and presented plaque. (G) Completed removal of plaque. (H) Incision suture. (I) Embolus was formed in carotid endarterectomy (CEA). (J) The thrombotic plaque disappeared after 0.5 mg tirofiban was administered to the artery. (K,L) Postoperative left ICA arteriogram revealed normal.
At the time of discharge, the patient was in stable condition, clear consciousness, mild aphasia, grade I muscle strength of the right upper limb and grade IV muscle strength of the lower limb.
A 48-year-old patient presented with the acute transient right upper limb
weakness two months later. Medical history was significant for hypertension, DM
and ischemic heart disease(IHD). Chest and abdominal CT scans, and an
echocardiogram. All the results were negative. At the first neurologic
examination a 90% stenosis of the ICA was diagnosed by Doppler study for more
than two months. Cerebral angiography showed 90% stenosis at the junction of
left ICA stenosis and about 50% stenosis at left vertebral artery (LVA)
stenosis. There was no associated nausea, vomiting, visual ghost, vertigo,
unconsciousness and dyskinesia. DSA showed stenosis of left ICA stenosis
(
Under general anesthesia, the left CCA, ICA and ECA, STA were exposed. After blocking up the artery, the anterior wall of the carotid artery is incised. The plaque presented as unstable, the intima of the ICA was carefully treated and the fragments were removed. Suture the carotid artery, leave a needle without ligation. The carotid sheath was sutured layer by layer, the right lower limb was braked, and the femoral sheath was fixed well. The muscle tension of limbs was normal. After operation, the patient had a head CT scan, which ruled out an ICH. DSA demonstrated that the left MCA was occlusion (Fig. 3). Then, through a 6-French introducer sheath in the RFA and after the administration of heparin (3000 U), a 6-French guiding catheter (Cordis Corporation, Bridgewater, NJ, USA) was advanced in the proximal left CAA. DSA show that embolus was formed in a position with a large angle of CEA on left ICA. The imaging of ICA was significantly improved after 0.5 mg tirofiban was administered to the artery.
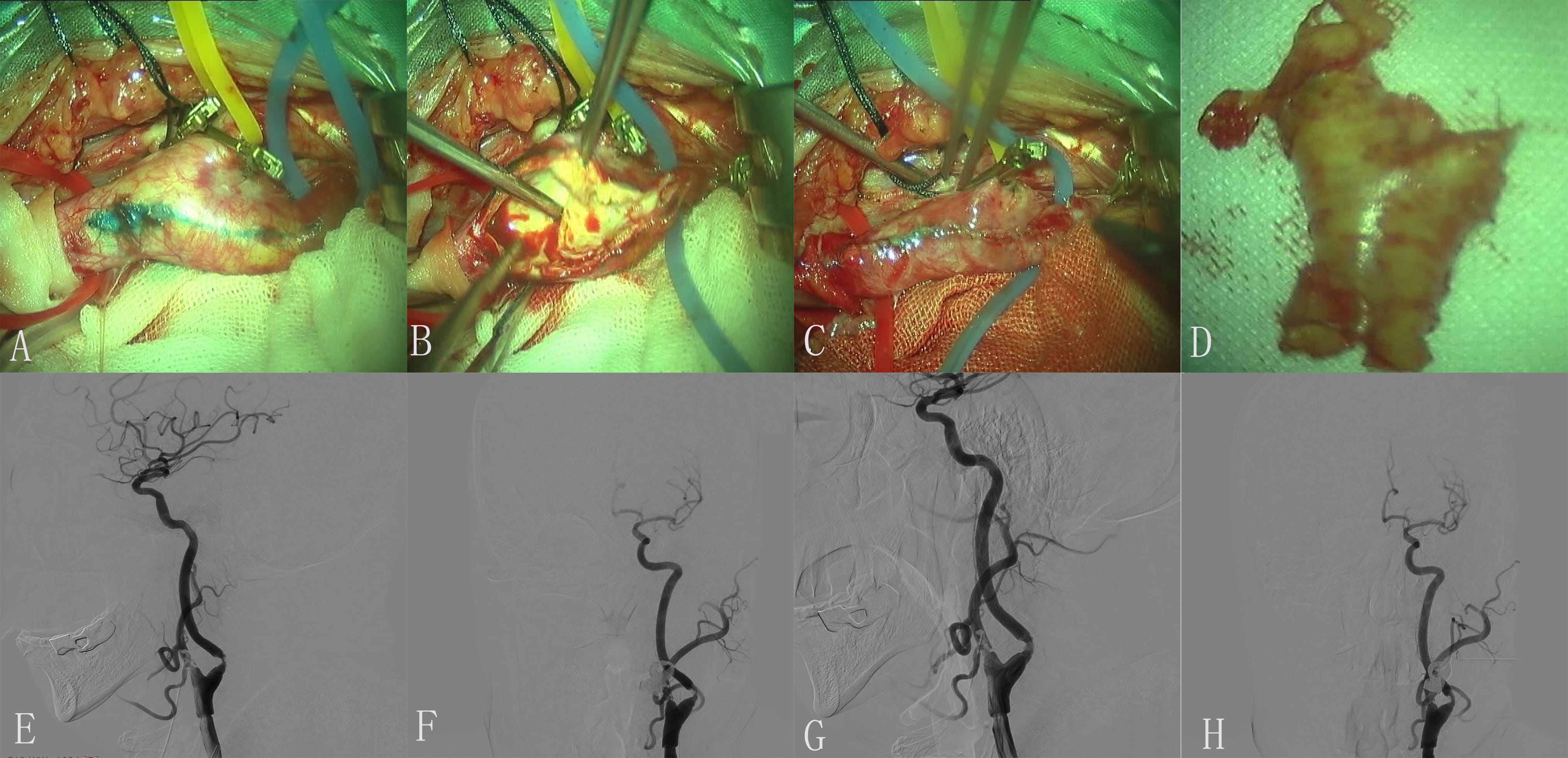 Fig. 3.
Fig. 3.Images of Case 3. Color Doppler ultrasonography of carotid artery on admission showed initial severe stenosis of the left internal carotid artery (ICA). The stenosis is greater than 90%. (A) Each branch was blocked by exposed responsibility carotid artery. (B) The endometrium of the common carotid artery (CCA) was dissected longitudinally and presented plaque. (C) Incision suture. (D) Intimal plaque. (E,F) Embolus was formed in a position with a large angle of carotid endarterectomy (CEA). (G,H) The imaging of ICA was significantly improved after 0.5 mg tirofiban was administered to the artery.
At the time of discharge, the patient was in stable condition. Physical examination: conscious clear, slightly clumsy speech, movement of the limbs, good healing of the postoperative wound, no signs of inflammation, no swelling.
Our results show that patients with acute ischemic events that occurred after CEA has an advantage in terms of functional recovery when treated with mechanical thrombectomy or arterial thrombolysis. In our result, time is the most important factor in the whole process. Meanwhile, rapid restoration of blood flow helps to improve the prognosis in patients with acute ischemic events treated with thrombolysis or mechanical thrombectomy. Although endarterectomy can effectively treat ICA stenosis, a certain proportion of complications still exist [5]. Therefore, these therapeutic measures can further reduce complications and improve the prognosis of patients.
However, the size of embolus shedding directly affects the size of the ridge plug caused by cerebral vascular occlusion. It directly affects the prognosis of patients with AIS. Therefore, different types of embolus may require different approaches [6]. Intra-arterial therapy can be broadly divided into the chemical dissolution of clots with locally delivered thrombolytic agents and clot retrieval or thrombectomy with mechanical devices. Although early randomized trials and subsequent meta-analyses showed a benefit of treatment with pro-urokinase or urokinase, their results are not directly applicable to current decision making about treatment because the control groups did not include intravenous alteplase, and mechanical approaches have largely replaced locally applied thrombolytic agents as first-line therapy [7]. Intra-arterial treatment in patients with AIS caused by a proximal intracranial occlusion of the anterior circulation was effective and safe when administered within 6 hours after stroke onset. The recent success of endovascular stroke treatment has heralded a new era in the management of AIS with significantly improved outcomes for patients. A large number of patients may be amenable to this new treatment and as the evidence expands, the number of patients eligible for mechanical thrombectomy continues to increase [8]. There are several different treatments for acute stroke embolic lesions. A series of studies conducted in Europe, North America, and Australia demonstrated the effectiveness of mechanical thrombectomy with stent retrievers in restoring blood flow in occluded vessels, with subsequent improvement in functional outcome and reduced mortality, compared to intravenous thrombolysis (IVT) with recombinant tissue plasminogen activator (rt-PA) [9]. This has led to a major shift in the management of patients with suspected emergent large vessel occlusion (ELVO), and mechanical thrombectomy is now recognized as the gold standard of care. It is therefore essential that clinicians and related health professionals are aware of the new methods and the impact they can have on a large number of patients [10]. Table 1 (Ref. [11, 12, 13, 14]) demonstrates some studies with postoperative therapy for cerebral thromboembolism complicates internal CEA.
| Study | Cases, n | Postoperative complication | Endarterectomy lesion | Postoperative therapy | Results |
| Perler et al., 2020 [11] | 1 | Thrombosis of MCA | Left ICA | Urokinase | The patient made a nearly complete neurologic recovery after 48 hours |
| Barr et al., 1995 [12] | 1 | Occlusive intraluminal thrombus within the proximal MCA | Right ICA | Urokinase | The patient neurological examination at that time was remarkable only for a new, mild, right upper extremity pronator drift and right side strength of 4/5 |
| Comerota and Eze, 1996 [13] | 1 | Thrombosis of ACA | Left ICA | Urokinase | A repeat arteriogram demonstrated patency of the left anterior cerebral artery, with complete clot dissolution and resolution of the right hemiplegia on awakening |
| Eckstein et al., 1995 [14] | 1 | Thrombosis of MCA | Left ICA | Urokinase | At discharge from the hospital, the patient was free of complaint. A last neurologic examination indicated only a slight weakness of a facial branch and signs of dysdiadochokinesia of the right arm |
| Abbreviations: CEA, internal carotid endarterectomy; ICA, internal carotid artery; MCA, middle cerebral artery; ACA, anterior cerebral artery. | |||||
In addition to the risk of acute ischemia, patients with carotid endarterectomy will also face the risk of intracerebral hemorrhage after intervention due to the invasive nature of the procedure and mandated use of antithrombotic therapy. Although we have no cerebral hemorrhage in these cases, there is still a risk of bleeding. Recent studies [15, 16, 17] have found that among patients receiving percutaneous coronary intervention (PCI), the academic research association high bleeding risk standard (ARC-HBR) provides a certain standard for identifying patients with high bleeding risk. The study of Gragnano’s team [15] shows that the ARC-HBR framework is less well for acute coronary syndrome (ACS) patients due to bleeding risk underestimation among no definite criterion. Meanwhile, female sex also may be an independent predictor for overall bleeding events after percutaneous coronary intervention [16]. The factors affecting intracerebral hemorrhage are still controversial. Therefore, the ARC-HBR criterion may provide reference for the risk factors after CEA in the future. However, any technique also has its own characteristics, embolisms in different conditions can be resolved in different ways, thereby reducing the risk of treatment, as well as improving the prognosis of patients. There are several preventive measures to minimize the frequency of postoperative complications associated with CEA. Our results show that these studies are a valuable guideline for improving the prognosis of these patients.
CLW, HZS and BJZ have made substantial contributions to design of the work and patient treatment process; YG, SCX, ZYJ, PW have made substantial contributions to the acquisition, analysis, or interpretation of data for the work; and CLW, BJZ, IG, OB have drafted the work and revised it critically for important intellectual content. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of CLW, and the protocol was approved by the Ethics Committee of the First Affiliated Hospital of Harbin Medical University, Harbin, China (approval number: 14A0002).
Thanks to all the peer reviewers for their opinions and suggestions.
This study was funded by Scientific Research Project of Health and Family Planning Commission, Heilongjiang Province of China (Grant no. 2018507).
The authors declare no conflict of interest.
All relevant raw data are freely available to any researchers who wish to use them for non-commercial purposes while preserving any necessary confidentiality and anonymity. The datasets are available on request to the corresponding author.
