Academic Editor: Antonio Mangieri
Optimal anticoagulation is critical for
successful extracorporeal membrane oxygenation (ECMO) to counterbalance the
activation of the coagulation system initiated by the blood-biosurface reaction
and mechanical stresses. Systemic anticoagulation is achieved mainly with
unfractionated heparin (UFH). Activated clotting time (ACT) is a widely used
laboratory parameter to monitor anticoagulation. The therapeutic range of ACT is
180–220 s. We investigated the effect of a lower target ACT (
Extracorporeal membrane oxygenation (ECMO) is a supportive therapy that is increasingly being used for patients with acute respiratory or cardiocirculatory failure refractory to medical therapy. However, high mortality and morbidity rates are noted among patients receiving ECMO, partly due to device-related complications. Adverse outcomes, such as bleeding and thromboembolism, are expected due to the nature of the ECMO circuit components. As blood comes into constant contact with non-endothelial biosurfaces, the coagulation cascade, complement system, platelets and von Willebrand factor, and the fibrinolytic system are activated. Also, the shear stresses of the circuit pump contribute to hemolysis [1]. Continuous anticoagulation monitoring is crucial during ECMO, and a fine balance must be maintained between potential risks of bleeding and thromboembolism.
Unfractionated heparin (UFH) remains the mainstay for continuous anticoagulation
therapy. The activated clotting time (ACT) and activated partial thromboplastin
time (aPTT) are most commonly used to monitor heparin level. Although therapeutic
anticoagulation during ECMO is defined by an ACT of 180–220 s [2], there is no
consensus presently regarding the ACT target and the guidelines vary across
different centers [3]. In cases of active bleeding and thrombocytopenia, the
heparin dose is typically modified, which may lead to favorable outcomes [4, 5, 6, 7, 8, 9, 10, 11].
In this study, we investigated the effect of a lower ACT target (
This is a retrospective study at a single center. After institutional review
board approval (2020AS0038), the data were retrospectively collected from the
electronic medical records. Patients aged
We used two different sets of ECMO circuits. One circuit comprised of a poly-methyl-pentene membrane oxygenator (PLS Quadrox, Maquet Cardiopulmonary, Hirrlingen, Germany), a centrifugal pump (Rotaflow, Maquet Cardiopulmonary, Hirrlingen, Germany), and recombinant human albumin and heparin-coated tubes (Bioline, Maquet Cardiopulmonary, Hirrlingen, Germany). The other circuit comprised of a poly-methyl-pentene membrane oxygenator (Terumo CAPIOX EBS, Japan), a centrifugal pump (SL 45, Terumo CAPIOX EBS, Japan), and biocompatible hydrophilic polymer surface coated tubes (Xcoating, Terumo, Japan).
During cannulation, a heparin bolus (30–50 units/kg body weight) was
administered at the discretion of the attending clinician. The ACT was measured
at the bedside using a portable Hemochron 401 device (Hemochron, ITC Medical,
Edison, NJ, USA) with HRFTCA510 tubes. After ECMO, the ACT was measured to be in
the guideline range of 180–220 s. The circuit was then managed either without
heparin or with low-dose continuous intravenous UFH. If the ACT was
Continuous variables are expressed as median and range or mean and standard
deviation. The descriptive statistics are expressed as count and percentage. The
chi-square test or Fisher’s exact test was used to compare the categorical
variables. The Student’s t-test or Mann-Whitney U-test was used to
compare the continuous variables. Univariate and multivariate logistic regression
analyses were used to identify the risk factors for successful weaning from ECMO
support and survival until discharge. The results were considered statistically
significant if the p-value was
A total of 43 ECMO cannulations performed from March 2017 to October 2019 were
included in the study. There were 14 patients with ACT
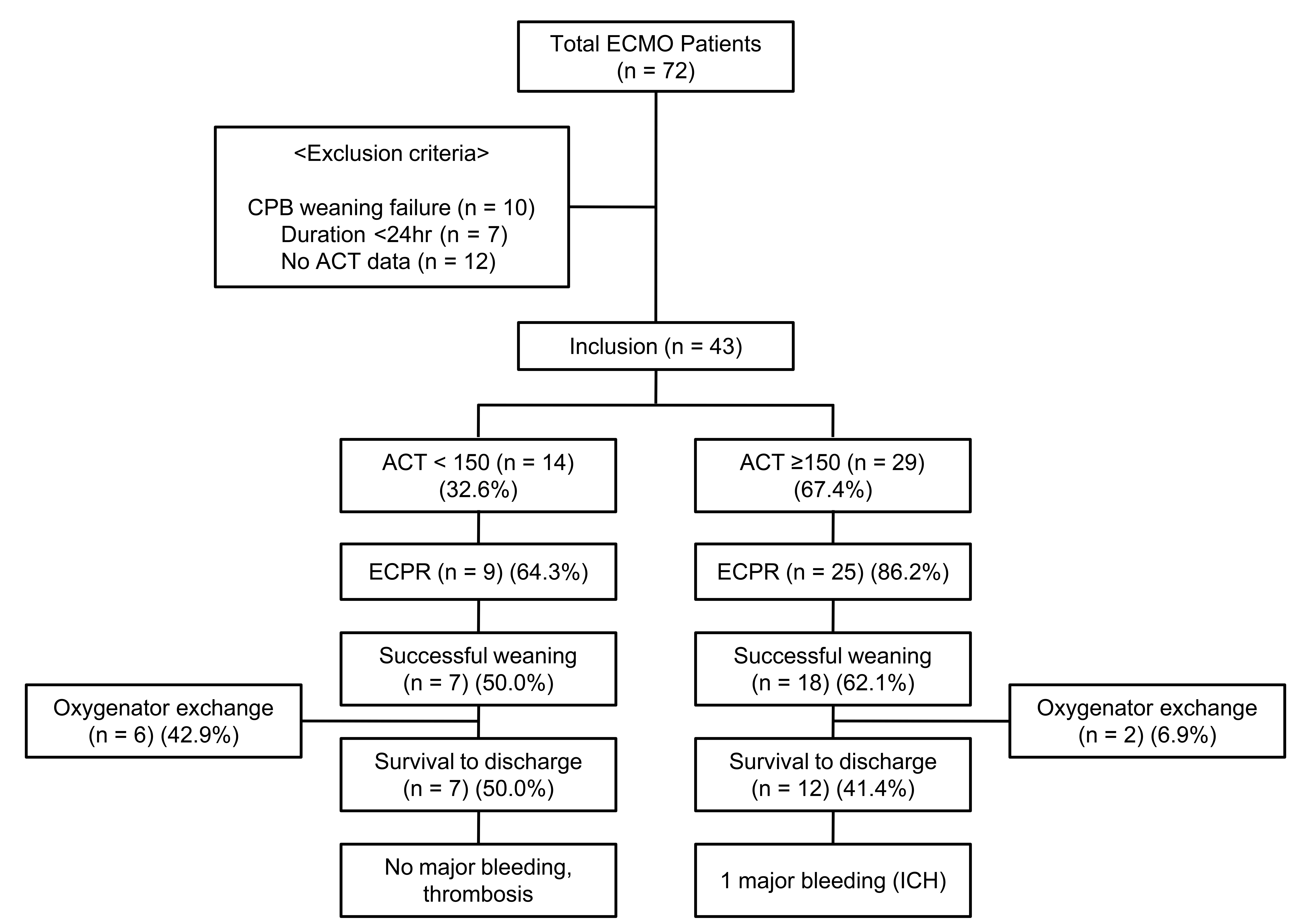 Fig. 1.
Fig. 1.Flow chart showing the inclusion and exclusion criteria for the study population. ACT, activated clotting time; CPB, cardiopulmonary bypass; ECMO, extracorporeal membrane oxygenation; ECPR, extracorporeal cardiopulmonary resuscitation; ICH, intracranial hemorrhage.
 Fig. 2.
Fig. 2.Difference in the mean ACT between the low ACT and conventional
ACT groups from ECMO day 1 to 7. ACT, activated clotting time; ECMO,
extracorporeal membrane oxygenation. * indicated p
There were no significant differences in the baseline characteristics of the two groups before ECMO (Table 1). There was a predominance of male patients in each group. The comorbidities included dyslipidemia (most common), hypertension, diabetes mellitus, chronic kidney disease, history of percutaneous coronary intervention (PCI), and history of cerebrovascular accident (CVA).
| Variables | Low ACT (n = 14) | Conventional ACT (n = 29) | p-value | |
| Age (years) | 59.6 |
57.2 |
0.611 | |
| Male (n) | 8 (57.1%) | 18 (62.1%) | 0.757 | |
| Comorbidities (n) | ||||
| Hypertension | 9 (64.2%) | 12 (41.4%) | 0.159 | |
| DM | 3 (21.4%) | 12 (41.4%) | 0.198 | |
| CKD | 1 (7.1%) | 2 (6.9%) | 0.976 | |
| Dyslipidemia | 9 (64.3%) | 15 (51.7%) | 0.437 | |
| h/o PCI | 3 (21.4%) | 7 (24.1%) | 0.844 | |
| h/o CVA | 3 (21.4%) | 5 (17.2%) | 0.741 | |
| ACT, activated clotting time; CKD, chronic kidney disease; CVA, cerebral vascular accident; DM, diabetes mellitus, ECMO, extracorporeal membrane oxygenation; PCI, percutaneous coronary intervention. | ||||
Table 2 shows the ECMO factors in each group. The VA and VV ECMO rates did not
differ in the low ACT and conventional ACT groups (9:5 vs. 22:7, p =
0.428). The proportion of patients receiving extracorporeal cardiopulmonary
resuscitation (ECPR) in the low ACT and conventional ACT groups was 64.3% (n =
9) and 86.2% (n = 25), respectively (p = 0.098). The frequency of use
of the PLS and EBS machines was similar between the low ACT and conventional ACT
groups (7:7 vs. 14:15, p = 0.916). The duration of ECMO support in the
low ACT and conventional ACT groups was 17.5
| Variables | Low ACT (n = 14) | Conventional ACT (n = 29) | p-value | |
| ECMO type (VA/VV) (%) | 9 (64.3)/5 (35.7) | 22 (75.9)/7 (24.1) | 0.428 | |
| ECPR (%) | 9 (64.3) | 25 (86.2) | 0.098 | |
| ECMO duration (days) | 17.5 |
20.7 |
0.102 | |
| ECMO machine (PLS/EBS) (%) | 7 (50.0)/7 (50.0) | 14(48.3)/15 (51.7) | 0.916 | |
| Average flow rate (L/min) | 2.7 |
2.9 |
0.819 | |
| Initial ACT (s) | 176.0 |
202.8 |
0.190 | |
| Mean ACT (s) | 139.8 |
181.4 |
0.001 | |
| Successful weaning (%) | 7 (50.0) | 18 (62.1) | 0.452 | |
| Survival to discharge (%) | 7 (50.0) | 12 (41.4) | 0.594 | |
| ECMO-related complications | ||||
| Oxygenator exchange | 6 | 2 | 0.009 | |
| Circuit clot | 1 | 0 | 0.145 | |
| Cannulation site bleeding | 1 | 0 | 0.145 | |
| Oral cavity bleeding | 0 | 1 | 0.145 | |
| Intracranial hemorrhage | 0 | 1 | 0.482 | |
| ACT, activated clotting time; ECMO, extracorporeal membrane oxygenation; ECPR, extracorporeal cardiopulmonary resuscitation; VA, veno-arterial; VV, veno-venous. | ||||
Of the 14 patients in the low ACT group, 7 (50%) were weaned off ECMO support, and all patients survived until discharge without further complications. Of the 29 patients in the conventional ACT group, 18 (62.1%) were successfully weaned off ECMO support and 12 (41.3%) survived until discharge. The cause of death was septic shock (n = 5) and intracranial hemorrhage (ICH; n = 1). There was no significant difference in the weaning rate (p = 0.452) and survival until discharge rate (p = 0.594) between the two groups. We also investigated patient outcomes according to ECMO support type (VA vs. VV) (Table 3). There were no differences between the two groups, except for ECPR frequency (100% vs. 25%, p = 0.001, respectively) and initial ACT time (205.4 s vs. 164.8 s, p = 0.007, respectively).
| Variables | VA ECMO (n = 31 ) | VV ECMO (n = 12) | p-value | |
| ACT |
9 (29.0) | 5 (41.7) | 0.482 | |
| ECPR (%) | 31 (100) | 3 (25) | 0.001 | |
| ECMO duration (days) | 8.0 |
10.5 |
0.498 | |
| ECMO machine (PLS/EBS) (%) | 13 (41.9) /18 (58.1) | 8 (66.6)/4 (33.3) | 0.185 | |
| Average flow rate (L/min) | 2.9 |
3.3 |
0.309 | |
| Initial ACT (s) | 205.4 |
164.8 |
0.007 | |
| Mean ACT (s) | 167.6 |
168.3 |
0.785 | |
| Successful weaning (%) | 20 (64.5) | 5 (41.7) | 0.301 | |
| Survival to discharge (%) | 16 (51.6) | 3 (25) | 0.174 | |
| ECMO-related complications | ||||
| Oxygenator exchange | 5 | 3 | 0.585 | |
| Circuit clot | 0 | 1 | 0.279 | |
| Cannulation site bleeding | 0 | 0 | 1.000 | |
| Oral cavity bleeding | 1 | 0 | 1.000 | |
| Intracranial hemorrhage | 1 | 0 | 1.000 | |
| ACT, activated clotting time; ECMO, extracorporeal membrane oxygenation; ECPR, extracorporeal cardiopulmonary resuscitation; VA, veno-arterial; VV, veno-venous. | ||||
Of the 9 patients receiving ECPR in the low ACT group, 6 (66.6%) were weaned-off from ECMO support uneventfully, and patients of which all survived until discharge. Among the 25 patients receiving ECPR in the conventional ACT group, 16 (64.0%) were successfully weaned off and 10 (40.0%) survived to discharge. There was no significant difference in the weaning rate (p = 0.866) and survival until discharge rate (p = 0.169; Table 4).
| Variables | Low ACT (n = 9) | Conventional ACT (n = 25) | p-value |
| Successful weaning (%) | 6 (66.6) | 16 (64.0) | 0.866 |
| Survival to discharge (%) | 6 (66.6) | 10 (40.0) | 0.169 |
| ACT, activated clotting time; ECMO, extracorporeal membrane oxygenation; ECPR, extracorporeal cardiopulmonary resuscitation. | |||
The ECMO-related complications, such as oxygen exchange rate, intra-circuit clot formation, cannulation site bleeding or hematoma, gastrointestinal bleeding, and cerebrovascular accident (either ischemic or hemorrhagic) events, were assessed. The oxygenator exchange rate was significantly higher in the low ACT group than in the conventional ACT group (6 patients vs. 2 patients, p = 0.009). Five oxygenators were electively replaced due to decreased oxygen exchange capability which was calculated by measuring pre-oxygenator blood gas value minus the post-oxygenator. The other one was replaced due to a thrombus visible inside the oxygenator without change in the ECMO flow. Bleeding occurred in 3 patients in the conventional ACT group (preexisting heel wound, n = 1; oral cavity, n = 1; ICH, n = 1) and in 1 patient in the low ACT group (femoral artery cannulation site) who was permitted voluntary movement before heart transplantation 19 days after ECMO.
Optimal anticoagulation is critical for successful ECMO support. To the best of our knowledge, there are no randomized controlled studies on adequate anticoagulation strategies in patients at a high risk of bleeding during ECMO. According to the Extracorporeal Life Support Organization (ELSO) guidelines, the therapeutic anticoagulation range is defined as an ACT of 160–220 s, variable according to the analyzers. This is, however, not universally agreed upon. The guidelines suggest UFH titration and adjustment of the ACT goal range based on factors including patient bleeding, circuit clotting, or the measured anti-factor Xa level [2]. According to a recent international survey, the target ACT for VA and VV ECMO ranged from 140 to 220 s. The majority of the institutions used an ACT between 160 s and 200 s [3]. In this study, we report the safety and efficacy of a lower ACT in patients on ECMO support.
The rate of weaning from ECMO support and survival until discharge did not show a significant difference between the low and conventional ACT groups. All patients in the low ACT group and two-thirds of the patients in the high ACT group who were successfully weaned from ECMO support were discharged to home. A majority of the patients who did not survive after weaning died from septic shock. A patient in the high ACT group developed ICH during ECMO and died, despite being successfully weaned from ECMO support even after normalization of the coagulation status. Another patient with diabetes mellitus in the high ACT group had bleeding from the heel of the foot, which required electro-cauterization. In the low ACT group, no major thromboembolic event, such as stroke, pulmonary thromboembolism, or mesenteric ischemia was observed. A patient in the low ACT group developed cannulation site bleeding after 7 days of ECMO support. This patient was allowed awakening without mechanical ventilation and the arterial cannula site bleeding occurred while moving in bed. The bleeding finally stopped after heart transplantation and ECMO support was terminated after 19 days.
The oxygenator exchange rate was significantly higher in the low ACT group. Six patients required oxygenator replacement. A grossly visible clot formed inside the VV-ECMO circuit in one patient. In the remaining 5 patients, there was decreased oxygenation capability on post-oxygenator blood gas analysis. This may be due to the formation of microthrombi that affect the fibers of the oxygenator membrane.
Improvements in the ECMO equipment, including centrifugal pumps,
poly-methyl-pentene membrane oxygenators, and heparin-coated circuits aid in
reducing the incidence of thrombosis during and after heparin discontinuation.
With these improvements, systemic heparinization during ECMO may be reduced or
stopped as long as the blood flow is maintained at a high rate [4, 5, 6, 7, 8, 9, 10, 11]. In our
study, the average flow rate was 2.7
The concept of maintaining ECMO in cases of multiple trauma with severe bleeding
and ICH with heparin-free or heparin-sparing strategies is evolving [4, 5, 16, 17]. This concept is being accepted by most intensivists presently. However,
there are only a few reports on the feasibility of low ACT during VA ECMO [7, 9].
ECPR is usually associated with hypothermia, metabolic acidosis-induced
coagulopathy, anti-platelet medication, like clopidogrel or ticagrelor-induced
platelet dysfunction before PCI, and mechanical chest wall massage-induced
bleeding, like sternal or rib fracture or intra-pericardial bleeding. Sometimes,
it is accompanied by a cannulation site and mucosal bleeding and bloody
nasogastric tube drains. We attempted to maintain a lower ACT during ECPR because
of the higher tendency for bleeding. When initiating ECPR, we usually administer
a heparin bolus (30–40 units/kg) and check the ACT every hour until it reaches
~140 s before continuous intravenous infusion of heparin is
initiated. If the bleeding persists and there is unstable ECMO flow, we usually
do not initiate heparin infusion even when ACT is ~120–130 s.
After bleeding is stopped, we initiate intravenous heparin with caution to
prevent re-bleeding. As thrombocytopenia and platelet dysfunction are common in
ECMO patients [18], especially those treated with aspirin and clopidogrel, we
attempted to maintain a platelet count of
Compared with other studies [4, 5, 6, 7, 8, 9, 10, 11, 16, 17], our study did not include multiple trauma patients on VV ECMO support. In the low ACT group, all VA ECMO supports were performed with ECPR. According to Shoskes et al. [19], the rate of acute brain injury appeared to be higher in VA ECMO patients than VV ECMO. Among described studies, ischemic stroke was most prevalent, and rates were significantly higher in VA ECMO patients. ICH showed no significant difference between modes of ECMO. The mechanism of ischemic stroke in VA ECMO may be considered due to thromboembolism, which is more likely than in VV ECMO because of arterial cannulation [19]. Thus, systemic anticoagulation protocol should be designed accordingly in different modes of ECMO. In our study, there were no significant differences in complication outcomes in VA vs. VV ECMO groups (Table 3). Since our result showed that all VA ECMO patients in low ACT group were comprised of ECPR patients, the predisposition to bleeding in these patients is believed to have played a role against thromboembolism. ECMO component exchange due to clotting can be quickly performed when the ICU team is well-trained; however, major bleeding is harmful and difficult to treat [8]. In our series, oxygenator exchange was more common in the low ACT group, but the incidence of serious bleeding and thrombosis was not observed more commonly. Although we could not identify any risk factor for ECMO weaning, dyslipidemia was a risk factor for survival until discharge. A low ACT was not a risk factor for weaning and survival.
This study has several limitations. First, this is a retrospective analysis of a
relatively small sample population at a single center without protocolized
approach among the clinicians. Second, the lower target value of ACT (
Improvements in the ECMO circuits aid in reducing the incidence of thrombosis
and systemic heparinization may be reduced or stopped in patients at a high risk
of bleeding. In our study, a lower target ACT (
ECMO, extracorporeal membrane oxygenation; ECLS, extracorporeal life support; ECPR, extracorporeal cardiopulmonary resuscitation; UFH, unfractionated heparin; ACT, activated clotting time; aPTT, activated partial thromboplastin time; VV, veno-venous; VA, veno-arterial; PCI, percutaneous coronary intervention; CVA, percutaneous coronary intervention; CPR, cardiopulmonary resuscitation; INR, international normalized ratio; ICH, intracranial hemorrhage.
JIH and HJS designed the study. JIH and JH performed the study. JIH and HJS wrote the manuscript. HJS and JH reviewed the drafts. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
All participants provided informed consent for inclusion before participating in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Korea University Ansan Hospital (approval number: 2020AS0038).
The authors would like to express their gratitude to all those who provided assistance during the writing of this manuscript.
This study was supported by a Korea University grant (K1924931).
The authors declare no conflict of interest.
