Academic Editor: Peter A. McCullough
The Corona Virus Disease 2019 (COVID-19) has become an unprecedented global
public health crisis and a pandemic associated with vicarious psychosocial and
economic stresses. Such stresses were reported to lead to behavioral and
emotional disturbances in individuals not infected with the COVID-19 virus. It is
largely unknown if these stresses can trigger acute cardiovascular events (CVE)
in such individuals. Covid-19-neagtive adults presenting with acute myocardial
infarction (AMI), cerebrovascular accident (CVA), or out-of-hospital cardiac
arrest (OHCA) during the COVID-19 pandemic in Jordan from March 15, 2020 through
March 14, 2021 were enrolled in the study if they reported exposure to
psychosocial or economic stresses related to the pandemic lockdown. Of 300
patients enrolled (mean age 58.7
The Corona Virus Disease 2019 (COVID-19) caused by Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) has evolved to a global pandemic with devastating consequences. The rapid spread across the globe resulted in an accelerating and relentless massive public health crisis with unprecedented numbers of infected patients and deaths in a relatively short period [1, 2].
These significant threats of the pandemic necessitated shutdown of almost all human activities, travel bans and restrictions, lockdown, social isolation, stringent home quarantine, physical distancing measures and disruption of public health services [3, 4]. Clinical studies suggest that such measures, coupled with fear of contagion, may have a detrimental effect on human behavior and mental health of individuals not infected with the COVID-19 virus. A wide spectrum of psychosocial and economic stresses of various degrees and durations were reported [5, 6] to cause anger, fear, hopelessness, poor concentration and indecisiveness, deteriorating work performance, reluctance to work, and sleep disturbance [7, 8].
Psychosocial and economic stressors can trigger acute cardiovascular events (CVE), including acute myocardial infarction (AMI), acute cerebrovascular accident (CVA) and out-of-hospital cardiac arrest (OHCA) during times of natural (i.e., earthquakes) or man-made (wars) disasters [9, 10]. It is largely unknown if the COVID-19 pandemic-related psychosocial and economic stresses, lockdowns, and galvanization of all but essential services can trigger acute CVE among individuals non-infected with the virus [11].
Jordan confirmed its first COVID-19 case on March 2, 2020, and the country reported more than 750,000 cases as of May 20, 2021. A nationwide total lockdown was implemented on March 21, 2020 followed by various measure of easing or tightening the lockdown to date. The Jordan COVID-19 Atherosclerotic Cardiovascular Events Study (JoCORE) evaluated individuals who sustained acute CVE during the first 12 months of the pandemic, and reported exposure to the pandemic lockdown-related stressors prior to sustaining the acute CVE. None of these individuals was infected with the COVID-19 virus. The first 55 patients with AMI who were enrolled in the study were reported earlier [12]. The current study included all triggered events (AMI, CVA and OHCA) evaluated in the first year of the pandemic. We sought to determine the clinical profiles, the nature and frequency of the pandemic-related psychosocial and economic stressors, and the short term prognosis of these patients.
This multicenter cross-sectional study enrolled consecutive adult patients
(
The psychological impact of COVID-19 was measured using a hospital-based modification of the Impact of Event Scale-Revised questionnaire. This is a well-validated and self-administered set of questions used for determining the extent of psychological and economic impact after exposure to a public health crisis [13].
The diagnosis of AMI included ST-segment elevation MI (STEMI) and
non–ST-segment elevation MI (NSTEMI). STEMI was diagnosed by the presence of
cardiac ischemic chest pain, ST-segment elevation of
The triggers the patients were exposed to prior to the onset of acute CVE were divided into two categories; psychosocial and economic stresses. The former included loneliness, isolation, lockdown or quarantine, fear and uncertainty, fear of contacting COVID-19 infection or of death, anger, hopelessness disrupted sleep, lack of medical care or access, loneliness, death of a significant person, separation from loved ones, heavy meals and overeating, smoking binge and strenuous physical activity. Economic stresses included financial hardships such as bankruptcy, loss of job, and volatile income. Patients discharged alive from hospital were followed up two weeks later to enquire about symptoms of viral illness or a confirmed COVID-19 infection.
The relative frequencies of the two categories of triggering events (psychosocial or economic) reported by the patients, were compared during three phases in the first year of the pandemic. The initial interval of the pandemic was featured by a total lockdown and slow rise in the COVID-19 cases (March 15 to July 15, 2020), the second interval had an almost total lifting of the lockdown and low number of infected cases (July 16 to November 15, 2020), and the third interval featured by a more stringent lockdown and very high numbers of infected cases (November 16, 2020 to March 14, 2021).
The research protocol was approved by the institutional review board (ethics committee) in each participating hospital and all patients gave written informed consent.
Descriptive statistics are displayed as mean (
A total of 305 patients with acute CVE were asked to participate in the study, and of those, 300 agreed to be enrolled during the 12-month period of the study, including 269 (89.7%) patients with AMI, 15 (5.0%) patients with CVA, and 16 (5.3%) patients with OHCA. Among the patients with AMI, 115 (42.8%) had STEMI and 154 (57.2%) had NSTEMI. All patients who had AMI or CVA tested negative for COVID-19 infection using the nasopharyngeal swab PCR testing at hospital admission. None of the patients who had OHCA were reported to have symptoms of viral illness, or were close contacts of COVID-19 patients in the one-month period prior to the event. Baselines clinical profiles of all patients and the three subgroups of acute CVE are depicted in Table 1. The majority of the patients (81.7%) were men, and one-fourth were 50 years of age or younger. The vast majority of the patients (91.7%) had at least one of the four classical cardiovascular risk factors (hypertension, type 2 diabetes, hypercholesterolemia and cigarette smoking), and only 8.3% of the patients did not have any of these risk factors. Patients who had AMI had higher prevalence of past diagnosis of coronary artery disease (CAD) and coronary revascularization than those who presented with CVA or OHCA. Patients who had OHCA tended to be younger, were more likely to be men, and had higher prevalence of cigarette smoking compared with those who had AMI or CVA.
| Clinical features | All patients | AMI patients | CVA patients | OHCA patients | p-value | |
| (N = 300) | (N = 269, 89.7%) | (N = 15, 5.0%) | (N = 16, 5.3%) | |||
| Mean age |
58.7 |
57.8 |
65.2 |
55.3 |
0.07 | |
| Age |
80 (26.7%) | 72 (26.8%) | 3 (20.0%) | 5 (31.3%) | 0.77 | |
| Men | 245 (81.7%) | 224 (83.3%) | 7 (46.7%) | 14 (87.5%) | 0.001 | |
| Hypertension | 151 (50.3%) | 132 (49.1%) | 14 (93.3%) | 5 (31.3%) | 0.001 | |
| Diabetes mellitus | 112 (37.3%) | 99 (36.8%) | 7 (46.7%) | 6 (37.5%) | 0.74 | |
| Dyslipidemia | 128 (42.7%) | 117 (43.5%) | 7 (46.7%) | 4 (25.0%) | 0.33 | |
| Cigarette smoking | 179 (59.7%) | 168 (62.4%) | 1 (6.7%) | 10 (62.5%) | 0.0001 | |
| Family history of premature CVD | 107 (35.7%) | 98 (36.4%) | 2 (13.3%) | 7 (43.8%) | 0.15 | |
| Past CAD | 105 (35.0%) | 102 (37.9%) | 1 (6.7%) | 2 (12.5%) | 0.007 | |
| Past CVA | 2 (0.7%) | 1 (0.4%) | 0 | 1 (6.3%) | 0.90 | |
| Past coronary revascularization | 69 (23.0%) | 68 (25.3%) | 0 | 1 (6.3%) | 0.02 | |
| Prior use of CV medications: | ||||||
| - Aspirin | 104 (34.7%) | 98 (36.4%) | 3 (20.0%) | 3 (18.8%) | 0.17 | |
| - Second APA | 52 (17.3%) | 47 (17.5%) | 3 (20.0%) | 2 (12.5%) | 0.84 | |
| - Statin | 97 (32.3%) | 93 (34.6%) | 3 (20.0%) | 1 (6.3%) | 0.04 | |
| - Beta blocker | 84 (28.0%) | 76 (28.3%) | 6 (40.0%) | 2 (12.5%) | 0.22 | |
| - RASi | 73 (24.3%) | 66 (24.5%) | 7 (46.7%) | 0 | 0.01 | |
| APA, antiplatelet agent; CAD, coronary artery disease; CVA, cardiovascular accident; CVD, cardiovascular disease; OHCA, out-of-hospital cardiac arrest; RASi, renin-angiotensin system inhibitor. | ||||||
Coronary angiography of 228 (84.8%) patients with AMI showed one vessel CAD in 130 (57.0%) patients, multivessel or left main CAD in 87 (38.2%), and spontaneous coronary artery dissection in one woman. Of those who had coronary angiography, percutaneous and surgical coronary revascularizations were undertaken in 210 (92.1%) and 18 (7.9%), respectively. All patients with CVA had thrombotic stroke. Carotid and cerebral arteriography in these patients showed small-vessel disease in 8 patients and spontaneous carotid dissection in one patient. None underwent endovascular intervention. Of the AMI and CVA patients, 6 (2.1%) had in-hospital death. None of the victims of OHCA was successfully resuscitated; 13 (81.3%) patients were pronounced dead at the collapse scene, and three (18.7%) were pronounced dead in the emergency department. None of the patients discharged alive from hospital developed symptoms of viral illness or a confirmed COVID-19 infection up to two weeks after the CVE.
Table 2 shows the triggering events the patients were exposed to prior to sustaining the acute CVE. Exposure to these triggering events was reported to extend for variable durations of time ranging from few days to several weeks prior to the occurrence of the CVE. None of the patients reported exposure to similar triggering events, significant vulnerability to stressful situations prior to the COVID-19 pandemic, history of neurological or psychiatric illness, or taking psychotropic medications. Five of the patients reported anxiety disorder in a sibling.
| Triggers | N | % | |
| Psychosocial stress | |||
| Loneliness, isolation and lockdown | 99 | 33.0% | |
| Fears | 68 | 22.7% | |
| Anger | 67 | 22.3% | |
| Disturbed sleep | 43 | 14.3% | |
| Death/sickness of a significant person | 20 | 6.7% | |
| Hopelessness | 16 | 5.3% | |
| Others | |||
| Economic stress | 157 | 52.3% | |
| Cigarette smoking binges | 58 | 19.3% | |
| Heavy physical exertion | 18 | 6.0% | |
| Eating binges | 10 | 3.3% | |
Exposure to a single triggering event was reported by 87 (29.0%) patients and exposure to two or more triggers was reported by 213 (71.0%) patients. Psychosocial stresses were reported more often than economic stresses (81.0% and 52.3%, respectively). More than 20% of the patients reported exposure to at least one of the psychosocial stresses including stress related to loneliness and lockdown, intense sense of isolation and lockdown, fears, and anger. Fears reported included fear of getting infected with the COVID-19 virus, fear of lack of medical care and fear and uncertainty of the future. Rarely reported stresses peculiar to the pandemic situation, not shown in the table, included stress of supervising children’s online learning, care of handicapped children due to day-care centers closure, and inability to attend father’s funeral due to lockdown.
The economic stressors included financial hardship, volatile income, and loss of job. Of patients who reported these stresses, 44.6% reported concomitant exposure to non-economic stresses as well. Excess food intake and smoking binges were reported by 3.3% and 19.3% of patients, respectively. The heavy physical exertion reported by 6.0% of patients was mainly related to carrying heavy weights during partial lockdown lifting and permitting shopping on foot.
Fig. 1 demonstrates the relative frequencies of psychosocial and economic stresses the patients were exposed to during three different time intervals in the first year of the pandemic. During the initial interval, 150 patients (50%) were enrolled, 79 patients (26.3%) were enrolled during the second interval, and 71 patients (23.7%) patients were enrolled during the third interval. The prevalence of psychosocial stress, but not of the economic stressors, showed significant swings during the three-time intervals. In the initial phase of the pandemic, psychosocial stresses were reported by 56.0% of patients enrolled, economic stressors were reported by 12.7%, and both stresses were reported by 31.3% of patients. During the second interval with partial lockdown and lower numbers of COVID-19 cases, psychosocial stress rate dropped to 27.8% and the percentage of patients with economic stressors rose to 21.5%. During the third interval which was marked by resurgence of high COVID-19 case count and imposing more stringent lockdown measures, psychosocial stress frequency increased to 40.8% with no significant change in the prevalence of the economic stresses.
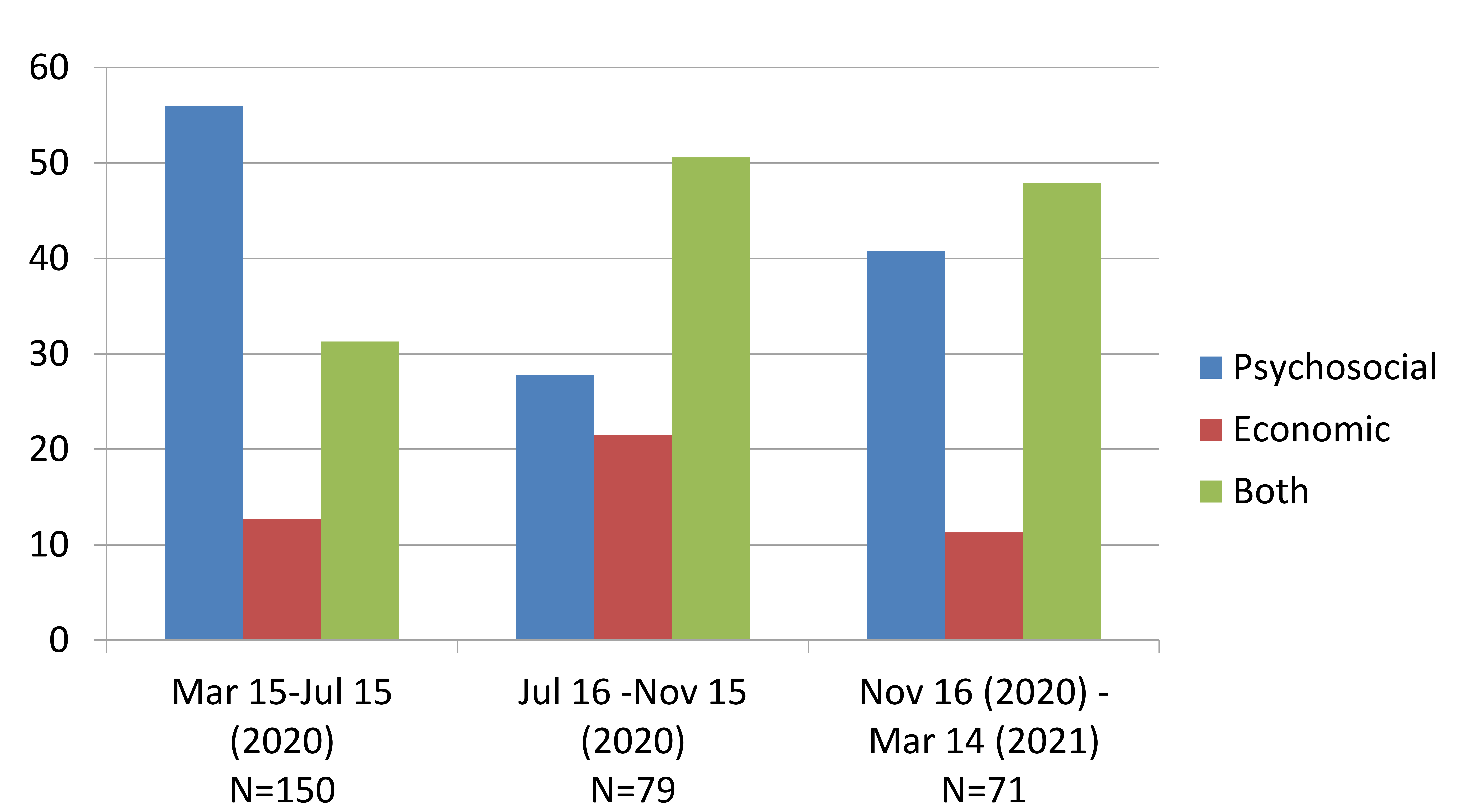 Fig. 1.
Fig. 1.Frequency of psychcosocial and economic stresses in 300 individuals during 3 different time intervals of the first year of the pandemic. p (ANOVA) = 0.0001 (psychosocial stress), 0.13 (economic stress), 0.006 (both stresses).
To our knowledge, this is the first study to demonstrate that acute CVE in individuals not infected with the COVID-19 virus can be triggered by the pandemic lockdown-related psychosocial and economic stressors. The mean age of the patients, and the prevalence rates of cardiovascular risk factors and preexisting CVD were comparable to those among patients with CVD in larger local studies [15].
Prior to the COVID-19 pandemic, studies have consistently demonstrated a temporal relationship of different respiratory viral epidemics and acute CVE, including AMI, CVA and OHCA, among individuals infected with these viruses [16, 17]. Individuals not infected with these viruses, however, were not reported to have acute CVE during the outbreaks, possibly due to the limited scales of virus spread and the fact that no lockdown measures were imposed [18].
In their efforts to control the spread of the COVID-19 virus and for the greater public benefit, governments imposed complete or partial lockdowns, mass quarantines, and shutting of all but essential services for various durations that extended from several weeks to months. These mandated regulations have led to a wide spectrum of lockdown-related psychosocial and economic stresses, including loneliness and boredom, fear of isolation, loss of freedom, frustration, anger, separation from loved ones, fear of COVID-19 infection or of medical care shortage, rapid spread of misinformation, uncertainty over disease status, loss of jobs, income volatility and financial hardships [19, 20, 21]. Such stresses led to mental and behavioral changes including anxiety, depression, sleep disturbance, and work inefficiency, but not acute CVE, among many individuals not infected with the COVID-19 virus.
The current study showed that psychosocial and economic stresses related to the COVID-19 pandemic could have created a combustible mix that triggered acute CVE in susceptible non-infected individuals, in a manner similar to triggered CVE associated with natural and human-made disasters [22, 23]. Such susceptibility may originate from a preexisting CVD or CV risk factors, inter-individual levels of tolerance to stress, and severity and length of exposure to the stress [24, 25]. Translating the impact of the pandemic-related triggers to a myocardial infarction involves interplay of several pathogenic events including increased sympathetic nervous system stimulation and circulating catecholamines leading to increases in heart rate, blood pressure and arterial wall shear force responses, increased coronary vascular tone, endothelial dysfunction, and enhanced pro-inflammatory and procoagulant mediators [26]. These responses may culminate in coronary plaque rupture and superimposed totally or subtotally occlusive thrombus in an epicardial coronary artery leading to AMI, ventricular tachyarrhythmias (VT) or cardiac arrest [27]. Similar pathogenic mechanisms in the extra- and intracranial arteries were shown to cause CVA due to exposure to triggering stressors [28, 29, 30]. Additionally, triggered events might be caused by spontaneous coronary or carotid artery dissection related to neurohormonal activation and vascular pathology [31, 32]. Furthermore, studies have shown that acute brain vascular insult and its accompanying sympathetic surge may adversely affect the cardiac function, producing echocardiographic, electrocardiographic, and enzymatic cardiac changes including cardiac systolic and diastolic dysfunction, ST segment abnormalities, various dysrhythmias, and elevations of N-terminal of the prohormone brain natriuretic peptide and cardiac troponins [33].
The great majority of the patients reported in this study (81.7%) were men. Studies have demonstrated the existence of gender disparities in patients developing coronary atherothrombosis and triggered acute CV events, with a documented advantage in premenopausal females. The cardioprotective roles of estrogen, which has been implicated as a major protective factor against atherosclerotic coronary plaque rupture, are mediated by the ability of this hormone to lessen the platelet aggregation and thrombosis while modulating timely pro- and anti-inflammatory responses to mitigate the possible fatal outcomes of the coronary occlusion. Such protective mechanisms are lost in women after menopause [34].
In concordance with other studies, psychosocial and economic stressors were the most common triggers reported during the COVID-19 pandemic [35, 36]. Some triggers may exert a single, sharp, and short transient effect on the pathophysiological process, such as a burst of anger capable of triggering an AMI, whereas other stressors, single or in combination, may exert pervasive effects over a longer period, such as frustration or financial hardship related to the pandemic lockdown [10, 27, 28].
Coexistence of triggers is common and includes, for example, anger related to loss of job, and sense of isolation due to fear of contracting the COVID-19 infection. The coexistence of multiple triggering factors makes it difficult to proportionally attribute the ensuing acute CVE to these multiple additive or synergistic effects of the stresses [31]. The current study also showed that psychosocial stresses were reported more often than economic burdens throughout the first year of the pandemic, but with significantly higher frequency during the periods featured by high COVID-19 case count and strict lockdown conditions. Despite an uneventful hospital course in the great majority of the AMI and CVA patients in this study, the prognosis of those who had OHCA was very grim.
Due to the increasingly arduous pandemic situation, it is unlikely that these triggering stressors will abate in the short term even after disappearance of the virus. On the long run and despite the gradual or partial lifting of the lockdown and drop in the COVID-19 case count, the sequelae of unresolved financial hardships, posttraumatic stress disorder, stress of lifting the lockdown and return to the usual life activities, could in turn continue to trigger acute CVE for longer periods [37].
Implementing preventive measures is essential to limit the occurrence of pandemic-related potentially life threatening complications. Knowledge of the vicarious traumatization experiences of individuals under lockdown or quarantine are critical to mitigate the negative impact of emotional and economic stressors, and to identify vulnerable individuals, with parallel development and implementation of mental health screening, timely and longitudinal preemptive interventions [38, 39, 40].
This study has few limitations. We cannot make a definite direct causal inference between the stressors and the acute CVE with a cross-sectional designed study. This study was not undertaken to evaluate the incidence of all triggered acute CVE during the lockdown period at a national level, thus limiting the generalization of our findings. It is plausible that there are large numbers of patients who sustained triggered acute CVE who were treated at other centers or did not seek medical advice. We acknowledge that the validity of reporting the exposure to various can be subjected to under- or over-reporting of events by the patient. However, several studies have shown an independent relation between self-reported stress intensity and the acute CVE [41].
The potential benefits of strict lockdown and limitation of human activity during the COVID-19 pandemic need to be weighed carefully against the possible psychological and economic costs. Our data show that the pandemic lockdown stressors are capable of triggering acute CVE in individuals not infected with the virus. Our findings need to be confirmed by larger studies from different parts of the world including our region to evaluate the prevalence, magnitude, and long term sequelae of the acute CVE triggered by lockdown-related stresses among non-infected individuals.
Study original idea and design were handled by AJH, IAA, and RT. Study feasibility and general supervision were worked on by AJH, EM, IAA, RT, HM, and YK. Statistical analysis and verification of the analytical methods by YK. Development of the case record forms and recruitment of investigators were managed by AJH, RT, OM and IAA. Contribution of cases and final manuscript writing were conducted by all authors. Data entry was made by AEM, AJH, OM and RI. Figure design and final drafting and manuscript drafting were handled by YK, RI, AJH, RT, OM and IAA. Results analysis and discussion were worked on by AJH, YK, IAA, and RT. Critical feedback and review of the manuscript were managed by AJH, OM, YK, RT, and IAA.
Approval codes of the Institutional Review Board (Ethics Committee) of the participating hospitals: Istishari Hospital (2020/7A JH), Farah Hospital (2020-FHospital-23Study), King Abdullah University Hospital (2020/KAHU-March23.56AW), Jordan University Hospital (2020-JHU2020/3J).
The authors would like to thank physicians who enrolled patients in the study (alphabetical order): Abdalla Al Harbi, Alhasan Saimeh, Ali Abu Romman, Ali Shakhatreh, Amr Karmi, Eyas Al-Mousa, Hadi Abu Hantash, Hesham Janabi, Ibrahim Jarrad, Ihab Algharabli, Islam Abu Sedo, Kamel Toukan, Mariana Noseir, Mohamad Jarrah, Mohammad Al Bakri, Mohammed Alqub, Mohammed Abu Shaban, Mohammad Jabari, Obada Mansour, Obada Zalat, Osama Okkeh, Qasem Shamyleh, Qusai Alzureikat, Raed Awaisheh, Walid Sawalha, Zaher Al Kasih, Zaid Alkhateeb, Zaid Khouri, and Ziena Abu Orabi.
This work was supported by a grant from Abdel Hameed Shoman Foundation Research Fund, the COVID-19 Studies 2020 Version (Grant 62020). The supporter had no involvement in the study design, data collection, analysis or interpretation of data, study conclusions or implications, or writing of the manuscript.
The authors declare no conflict of interest.
