Academic Editors: Brian Tomlinson and Takatoshi Kasai
Heart failure (HF) is a complex clinical syndrome resulting from the impairment
of ventricular filling or ejection of blood or both, leading to considerable
morbidity and mortality. Based on left ventricular ejection fraction (LVEF), the
2016 European Society of Cardiology (ESC) guideline firstly classified patients
with LVEF in the range of 40% to 49% into heart failure with mid-range ejection
fraction. Since then, more and more clinical studies targeting HF with mid-range
ejection fraction emerged, indicating that they may benefit from similar
therapies to those with LVEF
Heart failure (HF) is a clinical syndrome resulting from the dysfunction of
ventricular systolic and/or diastolic function caused by various reasons, leading
to an increasing disease burden worldwide. The mortality rate of HF remains high,
with approximately 2–17% of HF patients died during hospitalization and
17–45% of HF patients died within 1 year after admission [1]. The prevention
and treatment of HF are of vital significance. The 2016 European Society of
Cardiology (ESC) guideline [2] divided HF into three categories based on left
ventricular ejection fraction (LVEF). For the first time, the concept of
HF with mid-range ejection fraction was formally introduced,
with the LVEF in the 40–50% range. Besides, patients with LVEF
The mechanism of HFrEF was that the initial myocardial damage triggers persistent myocardial injury and myocardial remodeling, which eventually leads to a decrease in cardiac output [4]. HFpEF represented a complex syndrome, secondary to many different but interacting pathophysiological processes [5]. The phenotype and mechanism of HFmrEF were complex, with many characteristics between HFrEF and HFpEF [4, 5]. Hence more comprehensive examination methods were needed for further analysis of HFmrEF. The emergence of more technologies is conducive to the analysis of patients with HF from different perspectives, such as speckle tracking echocardiography (STE), cardiac magnetic resonance (CMR) quantitative imaging, and new biomarkers, which can better distinguish the characteristics of different types of HF and contribute to diagnosis and prognosis. In this review, we summarized the research progress of HFmrEF in clinical characteristics, prognosis, and treatment, hoping to help cardiologists better evaluate and treat patients of HFmrEF.
Since the term HFmrEF was first proposed in 2016, many clinical trials emerged, suggesting an HFmrEF prevalence of 12–25% among the overall HF population, which was less than HFrEF but comparable to HFpEF (Table 1, Ref. [6, 7, 8, 9, 10, 11]). For etiology, Ischemic heart disease (IHD) was the leading cause of HF, while the proportion of IHD in HFrEF and HFmrEF was higher than HFpEF. A post hoc analysis of Trial of Intensified versus standard Medical therapy in Elderly patients with Congestive Heart Failure [7] demonstrated the proportion of IHD in HFrEF, HFmrEF, and HFpEF was 58.2%, 56.5%, and 31.3%, respectively, indicating HFmrEF was similar to HFrEF in ischemic etiology. This was also confirmed by other studies [6, 8, 11, 12, 13, 14]. Dilated cardiomyopathy (DCM) was another important cause of HF, which accounted for about 1/3 of the total number of patients, with HFmrEF and HFrEF significantly higher than HFpEF [7, 8, 14]. Differently, hypertensive heart disease and valvular heart disease were more common causes of HFpEF than the other two types [7, 9]. Therefore, in terms of etiology, HFmrEF was more similar to HFrEF than HFpEF (Fig. 1, Ref. [7, 8, 11, 12, 13]).
| Study | Year | Patient number | Follow up (years) | HFrEF (%) | HFmrEF (%) | HFpEF (%) | |
| Kapoor et al. [6] | prevalence | 2016 | 99825 | inhospital | 49 | 12.8 | 38.2 |
| mortality | 3.06 | 2.62 | 3.02 | ||||
| Rickenbacher et al. [7] | prevalence | 2017 | 622 | 2.2 | 65 | 17 | 18 |
| mortality | 38 | 42 | 39 | ||||
| Chioncel et al. [8] | prevalence | 2017 | 9134 | 1 | 59.8 | 24.2 | 16 |
| mortality | 8.8 | 7.6 | 6.4 | ||||
| Koh et al. [9] | prevalence | 2017 | 42061 | 3 | 56 | 21 | 23 |
| mortality | 28.7 | 27.9 | 33 | ||||
| Löfman et al. [10] | prevalence | 2017 | 40230 | 1 | 57 | 22 | 21 |
| mortality | 14.8 | 14.7 | 18.5 | ||||
| Lund et al. [11] | prevalence | 2018 | 7598 | 2.9 | 57 | 17 | 26 |
| mortality | 30 | 15.8 | 16.6 | ||||
| Abbreviations: HFrEF, heart failure with decreased ejection fraction; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction. | |||||||
 Fig. 1.
Fig. 1.Etiologies for HFrEF, HFmrEF and HFpEF. (A) 2017 Chioncel et al. [8]; (B) 2017 Lupón et al. [12]; (C) 2017 Rickenbacher et al. [7]; (D) 2018 Lund et al. [11]; (E) 2017 Tsuji et al. [13]. Abbreviations: HFrEF, heart failure with decreased ejection fraction; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; DCM, dilated cardiomyopathy; HT, hypertension; IHD, ischemic heart disease.
The prevalence of hypertension (HT) and atrial fibrillation (AF) in HFmrEF were higher than HFrEF [7, 8, 9, 11]. In contrast, the prevalence of diabetes mellitus (DM) and chronic kidney disease (CKD) was comparable in HFmrEF and HFrEF. Compared with HFpEF patients, the prevalence of DM, anemia, AF, chronic obstructive pulmonary disease, and CKD was lower in HFmrEF [7, 8, 9, 10]. Regarding prognosis, compared with HFpEF, the correlation between CKD and mortality seemed to be stronger in HFrEF and HFmrEF, which may be due to the close relationship between CKD and the later stage of HF [10]. Similar to CKD, the effect of DM on mortality seemed to be greater in HFmrEF and HFrEF [15]. In contrast, AF had a similar impact on prognosis among all three types of HF [16].
Kapoor et al. [6] firstly analyzed the situation of 99,825 in-hospital patients, indicating that HFmrEF had the lowest in-hospital mortality of 2.62% while HFrEF accounted for 4.06% and HFpEF accounted for 3.02%. With regard to long-term prognosis, emerging studies demonstrated that the mortality of HFmrEF was 7–42% with a median follow-up time ranged from 1 year to 3 years, which was similar to HFpEF and slightly lower than HFrEF [7, 8, 9, 10, 11]. Therefore, we should attach more importance to the identification and management of HFmrEF. In terms of HF hospitalization rate, HFmrEF was similar to the HFpEF but significantly lower than HFrEF [8] (Table 1).
LVEF was the basis for the classification of HF. Prior studies have shown that
when LVEF was less than 45%, every 10% decrease in ejection fraction increased
the risk of death by 39% [17]. However, LVEF in HF was not static but often
changed over time. Only about 1/3 of HFmrEF patients maintained a constant HF
classification during long-term follow-up [12, 18], while some of the remaining
patients converted to HFrEF (25–37%) and HFpEF (25–33%) [19]. An
observational study conducted by Lupón et al. [12] including 940
patients with baseline LVEF
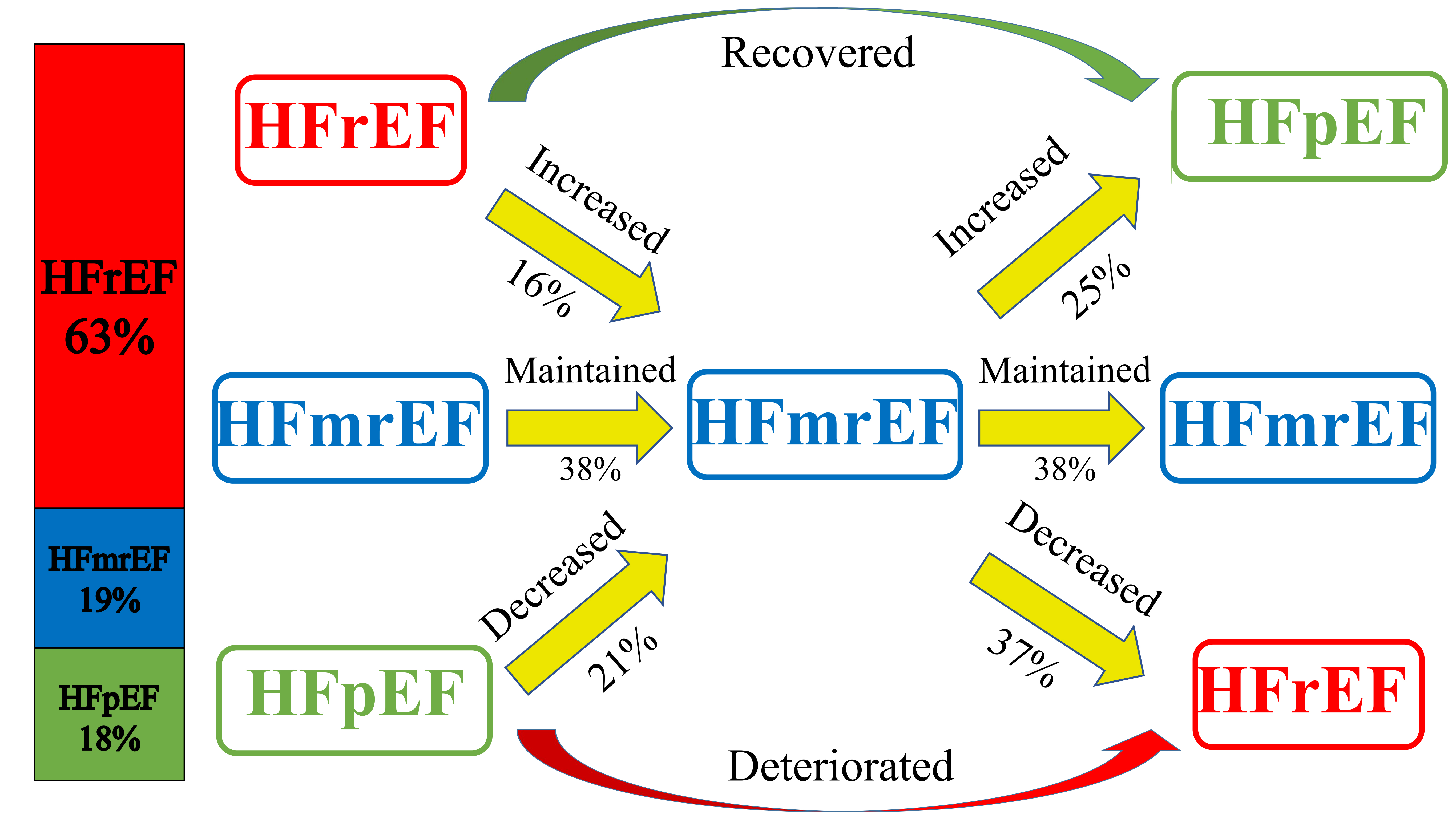 Fig. 2.
Fig. 2.Illustration of HF types changing with time. The bar chart on the left showed the proportion of the three types of HF. The figure on the right showed the transformation of different types of HF during follow-up [18]. Abbreviations: HF, heart failure; HFrEF, heart failure with decreased ejection fraction; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction.
When LVEF changed with time, the prognosis of patients seemed to be significantly different. Nadruz et al. [22] conducted a study including 277 patients with HFmrEF. They were divided into two groups according to the changes of LVEF:HF with mid-range ejection fraction and no recovered ejection fraction (HFmEF) and HFrecEF. The results showed that the incidence of composite end-point events of left ventricular assistant device implantation, heart transplantation, and all-cause mortality in the HFrecEF group was significantly lower than that in the HFmEF group (Hazard ratio [HR]: 0.31; 95% confidence interval [CI]: 0.15–0.67). And there was also a trend toward a lower risk of death (HR: 0.48; 95% CI: 0.22–1.05; p = 0.067) in analyses adjusted for all potential confounders. Lupón et al. [12] followed up the patients for 4.6 years, indicating the all-cause mortality and HF rehospitalization rates of HFrEF (HR: 1.74; 95% CI: 1.31–2.32) and HFpEF (HR: 1.83; 95% CI: 1.27–2.65) were significantly higher than HFrecEF, which demonstrated the prognosis of HFrecEF patients were better. A study conducted by Brann et al. [20] also showed that compared with patients whose LVEF improved from less than 40% to mid-range levels, patients whose LVEF deteriorated from greater than 50% had a higher risk of all-cause mortality and HF hospitalization (HR: 1.34; 95% CI: 1.10–1.82; p = 0.03) and cardiovascular mortality and HF hospitalization (HR: 1.71; 95% CI: 1.08–2.50; p = 0.02). Therefore, in patients with HFmrEF, the recovery of LVEF represented a better prognosis, while the deterioration of LVEF represents a worse prognosis, emphasizing the importance of early identification of this part of patients.
Although LVEF was used as the primary inclusion criteria in clinical trials to
determine effective drugs and devices for HF patients, this was mainly applicable
to HFrEF. And when LVEF was over 45%, the risk of end-point events was
relatively stable with the increase of LVEF [17, 23], indicating that LVEF had
limitations in assessing prognosis. The strain imaging technique has become a
powerful tool for accurately quantifying myocardial structure (including
longitudinal, circumferential, radial, and area strain) [24]. The global
longitudinal strain (GLS) was considered to be the most reliable parameter, which
mainly reflected the contractile function of the subendocardial layer of the left
ventricular wall [25] and was sensitive to early disease detection of left
ventricular dysfunction [24]. Moreover, GLS was closely related to the prognosis
of patients with HF. Chang et al. [26] conducted a retrospective study including 273 HFmrEF
patients who were divided into three groups in the follow-up period: HF with
worse EF (HFwEF) (LVEF
Sudden cardiac death (SCD) was one of the leading causes of death in patients
with HF, especially in patients with mild symptoms. Implantable cardioverter
defibrillator (ICD) implantation was an effective measure for preventing and
treating SCD. The 2021 ESC guideline [3] only regarded HFrEF as an indication for
ICD implantation and omit HFmrEF and HFpEF. Notably, although patients with LVEF
Myocardial fibrosis could be caused by a variety of pathological processes, and
its existence was related to poor clinical prognosis. CMR can provide a
non-invasive assessment of cardiac structure, function, and tissue
characteristics, among which late gadolinium enhancement (LGE) was one of the
most crucial examination methods for myocardial fibrosis. A prospective study by
Halliday et al. [31] including 399 DCM patients with LVEF
Although LGE was the gold standard for detecting myocardial fibrosis and evaluating myocardial viability, it can only be analyzed qualitatively and semi-quantitatively. CMR T1 mapping technique was a novel tool that allowed non-invasive quantitative analysis of intercellular and diffuse myocardial fibrosis [33]. The two most common measurements were native T1 and extracellular volume fraction (ECV).
Native T1 reflects the mixed signal of cardiomyocyte and extracellular stroma.
Diseases that lead to cardiomyocyte edema (such as acute myocardial infarction,
myocarditis) and extracellular interstitial volume increase (such as myocardial
fibrosis) can increase native T1. Doeblin et al. [34] demonstrated that
compared with healthy controls (972
Based on the T1 mapping technique, ECV was a new index calculated by obtaining
T1 value and hematocrit before and after injection of contrast agent, which used
a specific formula to reflect the percentage of extracellular interstitial volume
in the whole myocardial volume. Extracellular matrix expansion was a key factor
in ventricular remodeling and a potential therapeutic target, while ECV was a
marker of myocardial remodeling. A study by Treibel et al. [37]
including 1714 patients with HF, with a mean LVEF of 57% (45–64%),
demonstrated that ECV was independently associated with multiple end-points
including all-cause death and HF readmission (HR: 1.41, 95% CI: 1.26–1.59). And
there were 547 patients in the LVEF
CMR feature tracking (CMR-FT) technique used tissue voxel motion tracking on
steady-state free precession images to measure longitudinal, circumferential, and
radial left ventricular strain. Similar to STE, CMR-FT was rapid and
semi-automated, required no additional scans and sequences, and reduced
post-processing time. There was a good correlation between CMR-FT and two
dimensional/three dimensional-STE in GLS (r = 0.83 and 0.87) [38].
Furthermore, CMR-FT could avoid the poor image situation of STE effectively. In
different HF groups, GLS measured by CMR-FT was similar to that measured by STE.
The absolute value of GLS (–15.7%
HF causes cardiac pressure or volume overload, leading to the increased secretion of pro-B-type natriuretic peptide (BNP), which can be split into BNP, with the effect of not only decreasing angiotensin and aldosterone but also causing the vasodilation of arteries, and N-Terminal pro-BNP (NT-pro BNP) without any biological activity (Fig. 3). NT-pro BNP has been proved to be of diagnostic and prognostic value in patients with HFrEF and HFpEF [40, 41]. With respect to HFmrEF, an observational study involving 9847 outpatients with HF [42] showed that the median NT-pro BNP of HFmrEF patients was 1540 pg/mL, lower than 2288 pg/mL of HFrEF, and higher than 1428 pg/mL of HFpEF. Although NT-pro BNP in HFmrEF patients was lower than that in HFrEF patients, for prognosis, the risk of all-cause death and HF rehospitalization in HFmrEF patients with higher NT-pro BNP was twice as high as that in patients with lower NT-pro BNP (HR: 2.00; 95% CI: 1.71–2.34), higher than that in HFrEF (HR: 1.48; 95% CI: 1.36–1.61) and HFpEF (HR: 1.86; 95% CI: 1.58–2.18). For prognosis, the effect of elevated NT-pro BNP on HFmrEF was higher than that on HFrEF and HFpEF, which may be attributed to the “deterioration” of LVEF in some patients with baseline HFmrEF during follow-up.
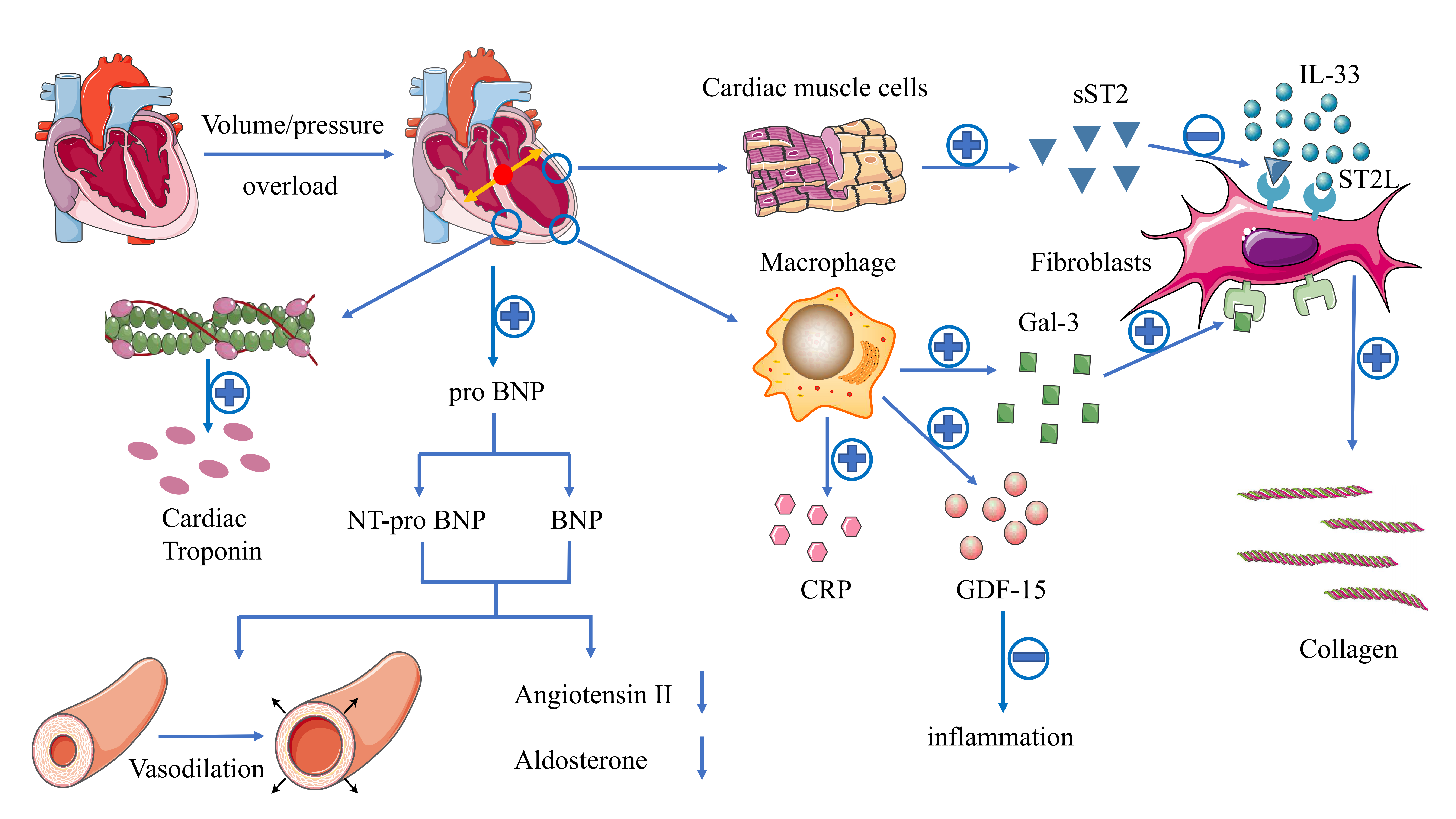 Fig. 3.
Fig. 3.Illustration of the mechanisms of different biomarkers in HF.
HF causes cardiac pressure or volume overload, leading to the increased
secretion of pro-BNP, which can be split into BNP, with the effect of not only
decreasing angiotensin and aldosterone but also causing the vasodilation of
arteries, and NT-pro BNP without any biological activity. ST2 is a member of the
IL-1 receptor family, mainly in the form of ST2L and sST2. IL-33 binds o ST2 to
produce anti-fibrosis and anti-apoptosis effects. However, under local
inflammation and/or mechanical or biochemical stress, the binding of sST2 to ST2L
in damaged myocardial tissue blocks the beneficial effects mediated by IL-33,
which leads to the decrease of resistance to apoptosis and the increase of
myocardial fibrosis. Gal-3 is a soluble
The cardiac troponin (cTn) complex consists of three subunits located on the filaments of the striated muscle, namely troponin T (TnT), troponin I (TnI), and troponin C (TnC). TnT is a protein that connects troponin complexes to tropomyosin, while TnI controls the binding of actin to myosin. Both TnI and TnT have been proved to be specific blood biomarkers of the heart (Fig. 3). The determination of high sensitivity cTn (hs-cTn) can provide a more sensitive measurement value so that a lower concentration can be detected. Moliner et al. [43] found no significant difference in hs-cTnT levels among the three HF types. But for prognosis, compared with HFrEF and HFpEF, the risk of all-cause death and hospitalization for HF in patients with HFmrEF was significantly higher (HR HFrEF vs. HFmrEF vs. HFpEF: 1.71 vs. 3.76 vs. 1.87), suggesting that patients with HFmrEF may be more sensitive to mild ischemic injury than patients with HFrEF or HFpEF (Table 2). Moreover, the simultaneous detection of hs-cTnT and NT-pro BNP can further identify high-risk patients [44]. In addition, in patients with normal NT-pro BNP, hs-cTnT was still independently related to the occurrence of adverse events, suggesting hs-cTnT can provide additional prognostic information on the basis of NT-pro BNP [45].
| Biomarker | HFrEF | HFmrEF | HFpEF |
| NT-pro BNP | ++ | ++ | ++ |
| sST2 | + | ++ | - |
| Gal-3 | + | ++ | ++ |
| GDF-15 | ++ | ++ |
++ |
| hs-cTn | + | ++ | + |
| hs-CRP | + | ++ | - |
| Abbreviations: HFrEF, heart failure with decreased ejection fraction; HFmrEF,
heart failure with mildly reduced ejection fraction; HFpEF, heart failure with
preserved ejection fraction; NT-pro BNP, N-Terminal pro-B-type natriuretic
peptide; sST2, soluble suppression of tumorigenicity-2; Gal-3, galectin-3;
GDF-15, growth differentiation factor-15; hs-cTn, high sensitivity cardiac
troponin; hs-CRP, high sensitivity C-reactive protein. | |||
Suppression of tumorigenicity 2 (ST2) is a member of the interleukin (IL)-1 receptor family, mainly in the form of the transmembrane binding receptor (ST2L) and soluble (sST2). IL-33 binds to ST2L [46] to produce anti-fibrosis and anti-apoptosis effects [47]. However, under local inflammation and/or mechanical or biochemical stress, the binding of sST2 to ST2L in damaged myocardial tissue blocks the beneficial effect mediated by IL-33, which leads to the decrease of resistance to apoptosis and the increase of myocardial fibrosis (Fig. 3). Therefore, sST2 is a new biomarker reflecting myocardial remodeling and fibrosis which could be used in the case of renal insufficiency [48]. An observational study conducted by Song et al. [49] compared the differences between sST2 among the three types of HF. The results showed that the sST2 levels of the three groups were similar (HFrEF vs. HFmrEF vs. HFpEF: 38.9 vs. 32.7 vs. 40.5 ng/mL; p = 0.194), which may imply that the degree of myocardial fibrosis may be similar in HF patients with different LVEF. In patients with elevated baseline sST2 levels, a sustained increase in sST2 levels during follow-up was associated with an increased risk of death [47]. Moliner et al. [43] assessed the relationship between sST2 concentration and prognosis in three HF types. The results demonstrated that sST2 had a significant prognosis effect in HFrEF and HFmrEF patients. But in HFpEF patients, the effect was not significant (Table 2).
Galectin-3 (Gal-3) is a soluble
Growth differentiation factor-15 (GDF-15), also known as macrophage inhibitory
cytokine-1 (MIC-1), belongs to the transforming growth factor-
C-reactive protein (CRP) has been identified as a classic marker of systemic inflammation, mainly produced by hepatocytes and cardiovascular tissue in the event of infection, cell invasion, or tissue injury [56] (Fig. 3). High sensitivity CRP (hs-CRP) can be detected at a concentration much lower than that of conventional CRP so that it can identify mild inflammation. A post-hoc analysis of PROTECT study [57] including 843 patients with acute HF, showed that CRP there was no significant difference among the three types of HF in patients with chronic HF. For prognosis, Moliner et al. [43] demonstrated that hs-CRP was independently associated with all-cause death and hospitalization of HF in HFmrEF and HFrEF (HR: 1.84 vs. 1.50) and had a higher prognostic significance for patients with HFmrEF compared with HFpEF. However, in patients with HFpEF, CRP cannot predict its prognosis, which was consistent with the results of previous studies [58, 59] (Table 2). Obviously, as a marker of systemic inflammation, CRP is likely to be affected by a variety of diseases other than HF, resulting in a decrease in its specificity.
In conclusion, since HFmrEF was proposed, more biomarkers have been used to evaluate HFmrEF. But it should be noticed that only natriuretic peptides and cardiac troponin were mentioned in the latest guideline in 2021 [3], which means that further research is still needed for the other biomarkers above.
At present, there are no clinical trials conducted solely for HFmrEF patients, and the current evidence comes from the subgroup analysis of clinical trials with HFpEF and HFrEF patients as the study population.
In conclusion, since the HFmrEF firstly proposed in 2016 ESC guideline, more and
more studies started to focus on this this subtype of HF. And benefits were found
in some conditions such as patients with sinus rhythm or CAD. Since the similar
etiologies and comorbidities with HFrEF, the 2021 ESC guideline [3] recommended
the use of
Since similar characteristics have been found between HFmrEF and HFrEF patients,
researchers started to confirm the treatment effort of HFrEF in HFmrEF patients.
The CHARM-preserved [64] and I-PRESERVE trial [65] which included patients of
LVEF
The same results were found in another drug recommended by guidelines to treat
HFrEF, the mineralocorticoid receptor antagonist (MRA). The TOPCAT trial [67]
including patients with LVEF
Similarly, the PARAGON-HF trial [70] including patients with LVEF
| Study | Type | Drugs | Control | LVEF | HR/RR | 95% CI | Outcome | p value |
| ACEI/ARB | ||||||||
| CHARM-preserved [64] | RCT | Candesartan | placebo | 0.89 | 0.77–1.03 | cardiovascular death + hospitalization for HF | 0.118 | |
| I-PRESERVE [65] | RCT | Irbesartan | placebo | 45–59% | 0.98 | 0.85–1.12 | all-cause mortality + hospitalization for cardiovascular cause | 0.28 |
| CHARM [11] | RCT | Candesartan | placebo | 40–49% | 0.76 | 0.61–0.96 | cardiovascular death + hospitalization for HF+ | 0.02 |
| PEACE (post hoc analysis) [66] | RCT | Trandolapril | placebo | 40–50% | 0.79 | 0.63–0.98 | all-cause mortality + nonfatal MI + stroke | 0.03 |
| Swede HF [9] | Registry | ACEI/ARB | - | 40–49% | 0.56 | 0.50–0.62 | all-cause mortality | |
| 0.63 | 0.54–0.75 | |||||||
| OPTIMIZED-HF [61] | Registry | no |
1.21 | 0.87–1.68 | mortality at 60–90 days | 0.255 | ||
| CHART-2 [13] | Registry | no |
40–49% | 0.57 | 0.37–0.87 | mortality | 0.010 | |
| SENIORS [62] | RCT | Nebivolol | placebo | all | 0.86 | 0.74–0.99 | all-cause mortality + cardiovascular hospitalization | 0.42 |
| Swede HF [9] | Registry | no |
40–49% | 0.74 | 0.59–0.92 | all-cause mortality | 0.006 | |
| 0.99 | 0.78–1.26 | 0.944 | ||||||
| meta-analysis [63] | placebo | 40–49% | 0.48 | 0.24–0.97 | cardiovascular death | 0.04 | ||
| MRA | ||||||||
| TOPCAT [67] | RCT | Spironolactone | placebo | 45–50% | 0.72 | 0.50–1.05 | all-cause death + hospitalization for heart failure | 0.046 |
| ARNI | ||||||||
| PARAGON-HF [70] | RCT | Sacubitril/valsartan | valsartan | 45–57% | 0.78 | 0.64–0.95 | cardiovascular death + hospitalization for heart failure | |
| SGLT2 inhibitor | ||||||||
| EMPEROR-Reduced [71] | RCT | Empagliflozin | placebo | 0.75 | 0.65–0.86 | cardiovascular death + hospitalization for HF | ||
| DAPA-HF [72] | RCT | Dapagliflozin | placebo | 0.74 | 0.65–0.85 | worsening HF + cardiovascular death | ||
| SOLOIST-WHF [73] | RCT | Sotagliflozin | placebo | 0.48 | 0.27–0.86 | total number of deaths from cardiovascular causes + hospitalizations and urgent visits for heart failure | - | |
| 0.72 | 0.56–0.94 | |||||||
| EMPEROR-Preserved [74] | RCT | Empagliflozin | placebo | 40–50% | 0.71 | (0.57–0.88) | cardiovascular death + hospitalization for HF | |
| 50–60% | 0.80 | (0.64–0.99) | ||||||
| Abbreviations: HFmrEF, heart failure with mildly reduced ejection fraction; MI, myocardium infarction; HF, heart failure; LVEF, left ventricular ejection fraction; HR, hazard ratio; RR, risk ratio; CI, confidence interval. | ||||||||
Sodium-glucose cotransporter 2 inhibitor (SGLT2i) was a newly
emerging drug used for improving glycaemic control in patients with DM by
decreasing renal glucose reabsorption and thereby increasing urinary glucose
excretion, which was found to be effective in HF patients recently [76]. The
EMPEROR-Reduced trial [71], which consists of patients with LVEF less than 40%,
demonstrated that empagliflozin reduced the primary composite outcomes of death
from cardiovascular causes or hospitalization for HF (HR: 0.75; 95% CI:
0.65–0.86; p
The SOLOIST-WHF trial conducted in 2020 including HFrEF, HFmrEF, and HFpEF
patients, showed that among patients with DM who had worsening HF, the end-point
events of the total number of cardiovascular deaths, hospitalizations and urgent
visits for HF were significantly lower with sotagliflozin than with placebo [73].
The results were still consistent in patients with both HFpEF (LVEF
Since the introduction of HFmrEF as an HF category in 2016 ESC guideline, a considerable number of clinical studies have included patients in the “grey area”, giving us a preliminary understanding of this field. HFmrEF represents not only the intermediate category between HFrEF and HFpEF but also the patient group with different clinical characteristics and prognosis. HFmrEF resembles a transitional stage between HFrEF and HFpEF, including HFwEF, HFsEF, and HFrecEF, which have different prognosis. Therefore, it is of great significance to find appropriate indicators to identify each type of HFmrEF.
In today’s era of precision medicine, new advances in HFmrEF treatment may be further extended to identify the characteristics of each HF patient, and these characteristics may help to further improve risk stratification. At present, cardiovascular imaging has developed from obtaining qualitative diagnostic information to more quantitative evaluation methods. Advanced imaging methods, including 2D-STE, 3D-STE, T1 mapping, and CMR-FT, et al., have been proved to have the potential to identify high-risk patients in HFmrEF, and the emergence of new biomarkers have been proved to provide additional predictive value. Multiple time points and combined detection of various indicators will also be the development direction of precision therapy in the future.
Conceptualization—WG; investigation—ZS; writing - original draft preparation— ZS; writing - review and editing—XW and WG; project administration—XW; funding acquisition—WG; All authors have read and agreed to the published version of the manuscript.
Not applicable.
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This work was supported by grants from the Peking University Health Science Center - University of Michigan Joint Institute for Translational and Clinical Research (grant No. BMU2020JI009), and the National Natural Science Foundation of China (grant No. 81972149, 81871850), Beijing Natural Science Foundation (grant No. 7212125).
The authors declare no conflict of interest.
