Academic Editor: Peter A. McCullough
Background: Disconnected unilateral pulmonary arteries are frequently misdiagnosed as “absent”. They typically arise from the base of the innominate artery and are fed by an aberrant arterial duct. If diagnosed early enough, they can be reconnected with catheter techniques even after closure of this aberrant duct. Consecutive surgical anatomical correction at a later stage is possible. Methods: Four cases illustrate the anatomical findings on computed tomography and angiography, all show an outpouching at the base of the brachiocephalic artery. Results: The therapeutic approach consisted of stenting of the aberrant ductus and consecutive surgery. In the oldest patient, 13 years, such an approach was impossible. Conclusion: If identified early in life, disconnected pulmonary arteries can be recruited with catheter techniques, and reconnected surgically at a later stage. It is not yet known if this approach prevents pulmonary damage, which is frequently seen in older untreated patients.
Disconnected pulmonary arteries are frequently misdiagnosed as “absent” [1]. Frequently these are initially fed by an aberrant ductus from the origin of the innominate artery, and become disconnected once the ductus closes [2]. On computed tomography (CT) or magnetic resonance imaging (MRI) they may not be well visible, as the flow is markedly reduced. Nevertheless, the ampulla of the aberrant ductus can frequently be identified. Pulmonary venous wedge angiography can help delineate the anatomy of the disconnected pulmonary artery, and determine the distance between the origin of the ductal ampulla and the hilus of the pulmonary artery (PA) [3].
Treatment may consist of primary surgery to reconnect the disconnected PA, or of initial catheterization to recanalise the arterial duct followed by surgery at a later stage [2, 4]. Both strategies can achieve growth of the PA.
If identified in early childhood, the aberrant arterial duct can be recanalised with stents, thus facilitating growth of the affected pulmonary artery [2, 5]. Surgical re-implantation onto the main pulmonary artery can then be achieved later [6, 7]. There is one report of a 10-year-old girl where this could be achieved too, but the majority of reported cases are small children [3].
Interestingly, in older children with this entity, abnormal lungs with bronchiectasis, recurrent pulmonary infections, hemoptysis and respiratory distress have been reported as early as 1982 [8]. It is not yet known if these changes can be prevented by early reconnection of the PA.
We present four cases of this entity, which illustrate anatomical and technical considerations.
Female ex-dysmature infant, birthweight 2800 g, 22q11 deletion. Right aortic
arch. Hypoplastic left lung. The initial diagnosis was “absent LPA”, as this
vessel was not visible on echocardiography or CT. However, on angio-CT an
outpouching was noted at the base of the left sided innominate artery (Fig. 1A).
On angiography at the age of 5 months, a tiny vessel could be seen towards the
LPA (Fig. 1B). This could be entered with a 0.014 balance middleweight coronary
wire through a 4F Cobra catheter (Cordis, Santa Clara, United States) followed by
an 0.014 Ironman wire (both Abbott, Chicago, United States), over which the
catheter was exchanged to a 5F Torqvue (Abbott, Chicago, United States). Two Onyx
stents (4.5
 Fig. 1.
Fig. 1.Patency of aberrant duct restored in an ex premature baby (patient 1, see text). (A) Computed Tomography, coronal view. LPA not visible. (B) Angiography. Right aortic arch. Catheter position within ductal ampulla. (C) Angiography after stenting the ductus: continuity with LPA. * Ductal ampulla at the base of the left sided innominate artery. LPA, left pulmonary artery.
Male 8 months weighing 8 kg. Echocardiographically, the RPA (right pulmonary artery) could not be seen.
On angio- CT, a hypoplastic right lung was diagnosed. The RPA could not be
visualized, but at the base of the right innominate artery (in a left aortic
arch) an outpouching was seen (Fig. 2A). Catheterisation at 9 months of age
showed a meshwork of tiny vessels originating from here. The distal RPA appeared
small (Fig. 2B). The aberrant ductus was entered through a RIM catheter (Cordis,
Santa Clara, United States) with a 0.014 balance middleweight coronary wire
followed by an 0.014 Ironman wire (both Abbott, Chicago, United States), and
sequentially dilated with a 2.5
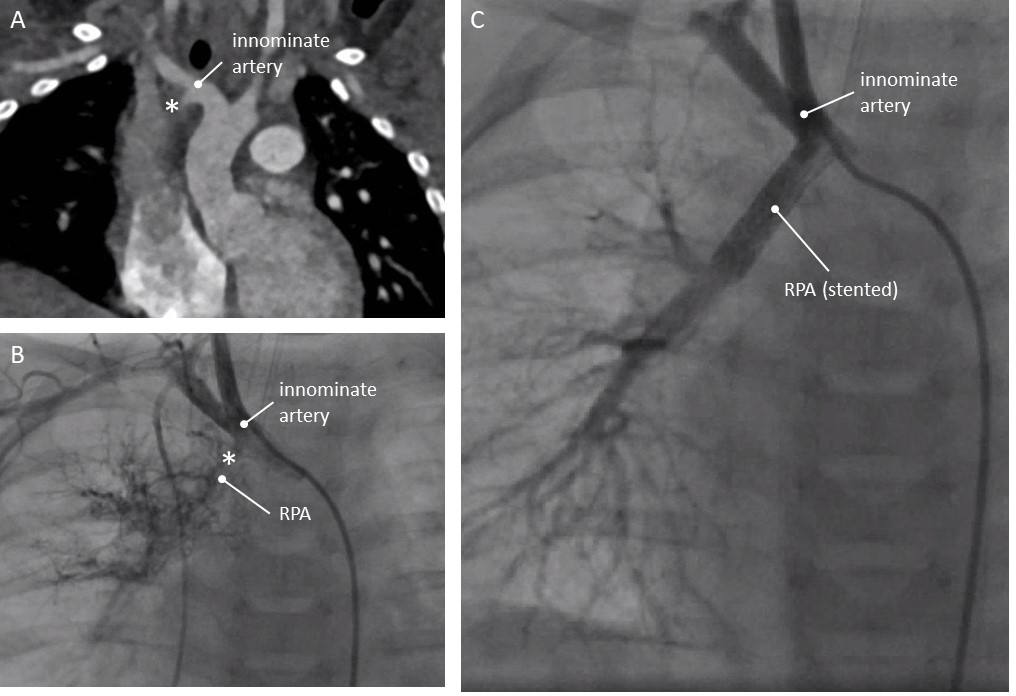 Fig. 2.
Fig. 2.Patency of aberrant duct restored in an 8 kg child (patient 2, see text). (A) Computed Tomography, coronal view. (B) Angiography. Left aortic arch indicated by catheter position. Angiography in the base of the innominate artery shows a small RPA. (C) Angiography after stenting the ductus: continuity with RPA. * Ductal ampulla at the base of the right sided innominate artery. RPA, right pulmonary artery.
Female neonate 2.85 kg. Resuscitation after birth. Thoracic drains were inserted
for a right sided pneumothorax. As the lungs did not expand immediately a CT scan
was performed. On CT the RPA could not be visualized, but at the base of the
innominate artery an outpouching was seen (Fig. 3A). Catheterisation was delayed
due to antibiotic treatment for an infection, and was carried out on day 10.
Through the foramen ovale, a wedge angiography was performed in the right
pulmonary veins. This confirmed the presence of the RPA (see
Supplementary Fig. 1). From the outpouching at the base of the innominate
artery a tiny tortuous aberrant arterial duct was seen (Fig. 3B). Through a 4F
Judkins right catheter (Cordis, Santa Clara, United States) coronary wires could
not be forwarded, but a 0.018 hydrophylic angled wire (Terumo, Shibuya, Japan)
straightened the duct reaching the RPA. A 0.014 GrandSlam wire (Asahi-intecc,
Tokyo, Japan) was positioned as a buddy wire, after which the Terumo wire was
removed carefully. Over the GrandSlam wire, two Rebel stents (Boston scientific,
Marlborough, United States) of 3.5
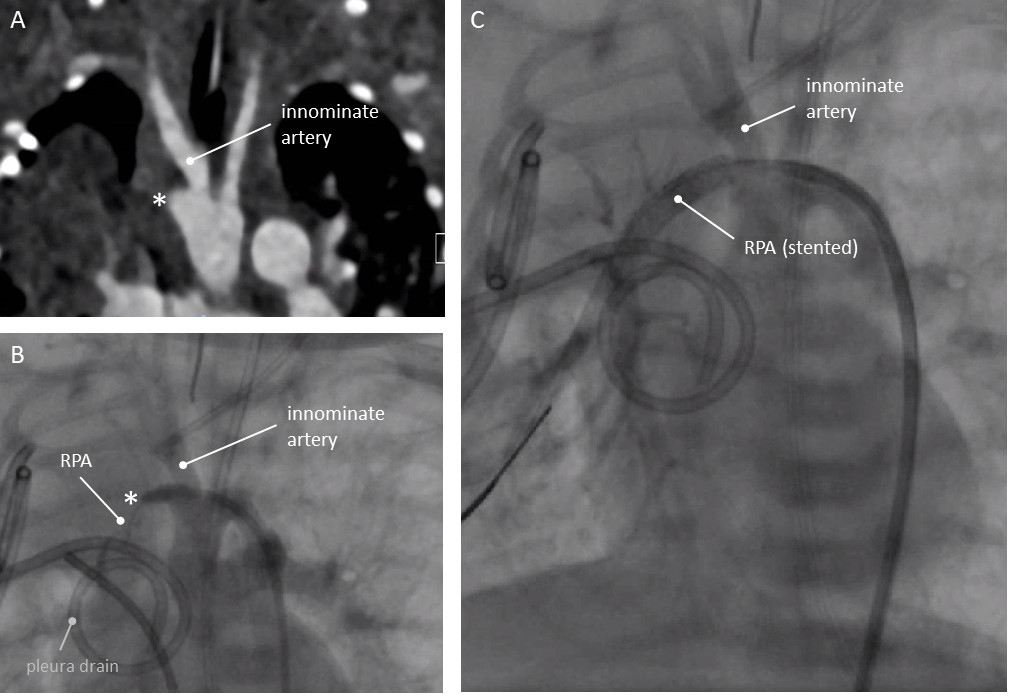 Fig. 3.
Fig. 3.Patency of aberrant duct restored in a neonate less than 3kg (patient 3, see text). (A) Computed Tomography, coronal view. No RPA visible. (B) Angiography. Left aortic arch indicated by catheter position. Catheter in the ductal ampulla. Small RPA discernable by direct injection into the ductus. Pleura drain in situ. (C) Angiography after stenting the ductus: continuity with RPA. * Ductal ampulla at the base of the right sided innominate artery. RPA, right pulmonary artery.
A 13-year-old female with right aortic arch. Since early childhood, she was diagnosed with a hypoplastic left lung and “absent left pulmonary artery”. On CT a possible small ductal ampulla at the origin of the innominate artery was seen (Fig. 4A). After extensive discussion, she underwent cardiac catheterisation. There was no connection from the origin of the innominate artery towards the left pulmonary artery. Via transseptal puncture, wedge-angiography into the left lower pulmonary vein was carried out and showed a very small caliber of the LPA without an obvious connection with the innominate artery (Fig. 4B). No intervention was carried out. Surgery was not considered feasible, as the distance from the MPA towards the remnants of the LPA was too large.
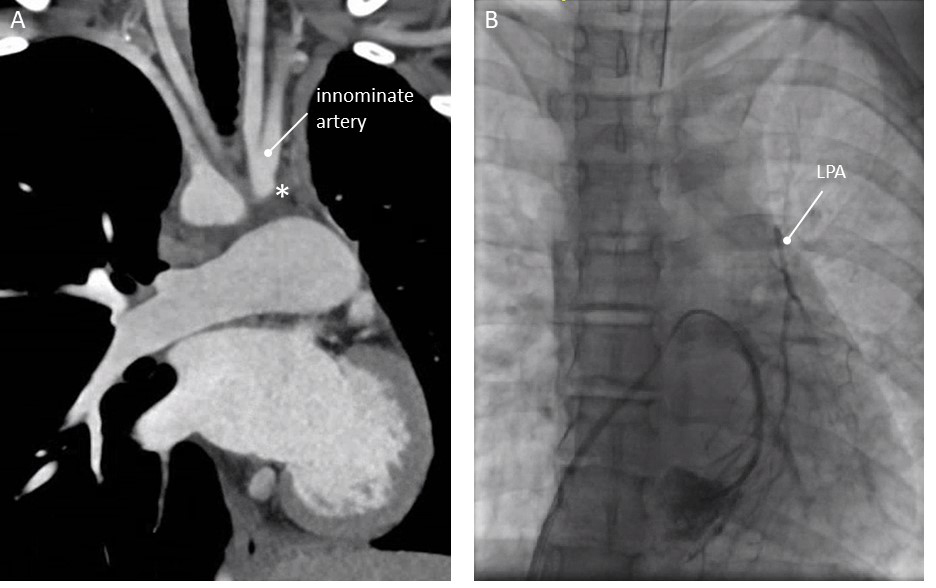 Fig. 4.
Fig. 4.Patency of aberrant duct not restored in 13 year old girl (patient 4, see text). (A) Computed Tomography, coronal view. (B) Wedge angiography with catheter in the left lower pulmonary vein. Hypoplastic, discontinuous LPA. * Ductal ampulla at the base of the left sided innominate artery. LPA, left pulmonary artery.
Patients diagnosed with “absent pulmonary arteries” rather have disconnected pulmonary arteries, which are frequently fed from an aberrant arterial duct arising from the origin of the innominate artery [1, 2]. In all our patients, the initial diagnosis was absence of one of the pulmonary arteries. In all of them, this diagnosis had to be revised to disconnected PAs. All patients had no additional congenital heart disease. The key finding on cross sectional imaging was a typical outpouching at the base of the innominate artery. Echocardiography allows identification of the sidedness of the “absent” pulmonary artery [9], but usually not of the aberrant, diminutive arterial ductus. The outpouching at the base of the innominate artery cannot be seen using echocardiography alone. Tian and Zheng relied solely on echocardiography [9], We advise additional cross-sectional imaging to facilitate subsequent catheter intervention as a first step to rehabilitate the disconnected pulmonary artery. Echocardiography alone is not sufficient to delineate the anatomy fully.
From a developmental stance, persistence of the distal sixth aortic arch with proximal involution has been proposed [8]. With closure of the arterial duct, the typical outpouching at the origin of the innominate artery remains [8]. We found this outpouching in all of our patients, and in three of them there was an aberrant arterial duct which could be recanalised.
If such a duct is recanalised early in life, the pulmonary arteries can be resurrected and re-implanted onto the pulmonary trunk [2, 3, 4]. We could achieve this in two of our patients to date; with a third awaiting surgery. Comparable strategies have been described earlier [10] and are still applied [11]. In case of stenosis of the reimplanted vessel, percutaneous treatment can be performed.
Although there is one case report of recanalisation of the feeding aberrant ductus in a 10-year-old child [3], this may not always be possible at older age. This underscores the importance of early diagnosis and intervention. Recognition of this entity is important, as it can be detected antenatally [12]. Postnatal management can then be planned adequately.
Although there are case reports and case series, it is not yet known if early treatment can prevent later lung pathology. Hence, further follow up of patients treated early in childhood is important.
If a unilateral pulmonary artery seems “absent”, cross sectional imaging should be performed. If an outpouching at the base of the brachiocephalic artery is seen this is most likely the ampulla of a former aberrant ductus arteriosus. In early childhood, and sometimes beyond, this can be recanalised as a first step, followed by corrective surgery later.
TK, MD, IB and GB—carried out all cardiac catheterisations; AB—carried out cardiac surgery; TK—drafted the manuscript, which was critically reviewed by all authors.
The study fulfills the ethical requirements of the Declaration of Helsinki. According to Nethlerlands law the need for written consent was not mandatory for this pure retrospective description.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
