Hypertensive
disorders of pregnancy (HDP) encompass a group of diseases. Single nucleotide
polymorphisms (SNPs) are common in the matrix metalloproteinase 9 (MMP9) genes.
The objective of this study was to analyse whether genetic polymorphisms in MMP9-1562 C/T alter the risk of HDP. Studies published up to October 2019 across
PubMed, ScienceDirect, SpringerLink, and China National Knowledge Infrastructure
database were searched. Case-control or cohort studies involving subtypes of HDP
and distribution of genotypes and/or alleles within MMP9-1562 C/T in both patients
and controls were selected. Number of genotypes and/or alleles for MMP9-1562 C/T
polymorphisms were obtained and analyzed using Stata software. Eight
published reports including 1300 HDP patients and 1612 controls were included in
the meta-analysis. Results showed that a variant genotype and allele of
MMP9-1562 C/T increased the risk of HDP,
with pooled OR 1.50 (95% CI 1.16–1.95, P = 0.002) and 1.36 (95% CI
1.15–1.61, P
Hypertensive disorders of pregnancy (HDP) are one of the common complications occurring during pregnancy, including gestational hypertension (GH), preeclampsia (PE), eclampsia, superimposed pre-eclampsia, and chronic hypertension in pregnancy [1, 2, 3]. The worldwide prevalence of HDP varies from 5% to 10% in pregnancies that cause significant maternal and fetal morbidity and mortality [4]. Though the exact cause and pathogenesis of HDP have not been identified, it is widely considered that the diseases are multifactorial and result from the interplay between multiple genetic, immunologic, and environmental factors (for example ambient air pollution) [2, 5]. Impaired cytotrophoblast invasion and placental ischemia followed by systemic endothelial dysfunction and hemodynamics disorder are thought to play a key role in the development of the disease [6, 7].
Placentation involves trophoblast invasion through the uterine decidua with extracellular matrix degradation and remodeling [8]. The family of matrix metalloproteinases (MMPs) are structurally related; zinc-dependent enzymes that have ability to degrade and restructure the extracellular matrix by activating the secretion of collagenases, gelatinases, and proteolytic enzymes [9]. Also, it has been reported that the imbalance between MMPs and their inhibitors, tissue inhibitors of metalloproteinases (TIMPs), plays a significant role in various adverse events such as high blood pressure [10, 11]. As a member of the MMP family, high-level expression of MMP9 is involved in the pathophysiology of trophoblast invasion [12, 13]. Single nucleotide polymorphisms (SNPs) are common within the MMP9 gene, and act as markers of disease susceptibility because of their potential to influence MMP-9 expression [14].
The relationship between susceptibility allele MMP9-1562 C/T (rs3918242) and the risk of HDP including GH and PE has been widely reported; however, the reported association between them has remained controversial [6, 15]. Two meta-analyses including six publications were performed respectively in 2014 and 2015 to evaluate the association between MMP9-1562 C/T polymorphisms and the susceptibility to PE [16, 17]. However, susceptibility to GH was not been included in these studies or in more recent studies (i.e., Leonardo et al., 2015; Sun et al., 2016) [18, 19]. Given the inconsistent nature of these findings, it is essential to systematically analyse whether genetic polymorphisms in the MMP9-1562 C/T alter the risk of HDP during pregnancy. This study was aimed at analysing whether genetic polymorphisms in MMP9-1562 C/T alter the risk of HDP.
We conducted a primary electronic search of the PubMed database for studies published up to October 2019. China National Knowledge Infrastructure (CNKI) database were also searched as a supplementary source of data. The search was performed using combinations of the following search terms without any limits: “matrix metalloproteinase-9”, “MMP-9”, “gestational hypertension”, “preeclampsia”, “eclampsia”, and “hypertensive disorders of pregnancy”. References were checked to identify repeated literature.
For selection in the meta-analysis, each study was required to meet all of the following inclusion criteria: (1) clinical subtypes of HDP including GH, PE, eclampsia, superimposed pre-eclampsia, and chronic hypertension in pregnancy were defined according to guidelines of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy [20]; (2) the study must have been either a case-control or cohort study; (3) the distribution of genotypes and/or alleles within the MMP9-1562 C/T is provided for both patients and controls; and (4) demographic data including participant characteristics such as country/ethnicity, disease subtypes, maternal age, and gestational weeks must be indicated.
Exclusion criteria were as follows: (1) case reports, guidelines, review articles, meta-analyses, mechanistic studies, and unrelated studies; (2) a lack of normal pregnant women as controls; (3) no reporting concerning MMP9-1562 C/T polymorphisms associated with susceptibility to HDP; (4) studies without performing consistency tests of genotype frequencies with the Hardy-Weinberg equilibrium (HWE) model; (5) duplicate data presented in multiple studies; (6) studies about the expression of MMP-9 protein, and (7) necessary data was unavailable.
Two authors independently evaluated the quality of the selected studies according to the Newcastle-Ottawa Scale (NOS) [21]. A total of three aspect parameters, including selection, comparability and exposure assessment, were assessed for each study. It was decided that a NOS score of 1–3 would indicate a “low-quality study”, a score of 4–6 a “moderate-quality study” and a score of 7–9 a “high-quality study” [22, 23]. Disagreements in quality assessment were resolved by discussion between the two authors.
The following information was extracted from each study: first author, publication year, country and primary race of the patients, classification diagnosis of HDP, sample size, maternal age, gestational weeks, consistency tests of genotype frequencies with the HWE model, and the distribution of genotypes and/or alleles within the MMP9-1562 C/T in the cases and controls. Two authors analyzed and extracted the data independently. Any disagreements were resolved by discussion.
The odds ratio (OR) and 95% confidence interval (95% CI) were used to
calculate the risks of HDP with MMP9-1562 C/T polymorphisms [24]. An OR
The electronic search was performed in the PubMed database and a total of 149 potentially relevant articles were identified. Additionally, China National Knowledge Infrastructure (CNKI) database were also searched for a supplementary search. And only one potentially relevant article was included. Therefore, a total of 150 of potentially relevant articles were evaluated. Per the inclusion and exclusion criteria, 8 articles meet our criteria for inclusion [6, 14, 15, 18, 19, 26, 27, 28]. It included 7 reports concerning PE, 2 reports concerning GH, and 1 concerning HDP without definitive disease classification (Fig. 1). Within these selected manuscripts, a total of 1300 patients with HDP and 1612 controls were included in the meta-analysis. In the included reports, 9 (90%) were high-quality and 1 (10%) was moderate quality. Detailed information concerning first author, publication year, country and primary race of the patients, classification diagnosis of HDP, numbers of cases and controls, maternal age, gestational weeks, whether have performed HWE, quality assessment of the selected studies, number of genotypes and/or alleles for the MMP9-1562 C/T in the case and controls are described in Table 1.
 Fig. 1.
Fig. 1.Flow diagram of the selection in the study.
| First author | Published Date | Country/ | Diagnosis | Cases/ | Maternal Age (cases vs controls) |
Gestational weeks (cases vs controls) |
HWE | NOS | MMP9-1562 C/T | MMP9-1562 C/T | ||||||||
| Ethnicity | Controls | Genotype (n) | Allele (n) | |||||||||||||||
| (n) | Case | Control | Case | Control | ||||||||||||||
| TT | TC | CC | TT | TC | CC | T | C | T | C | |||||||||
| Sun-PE | 2016 | China/ | PE | 107/242 | 28.85 |
27.75 |
Y | 7 | 7 | 35 | 65 | 11 | 53 | 178 | 49 | 165 | 75 | 409 |
| Asian | 26.85 |
26.82 | ||||||||||||||||
| Leonardo-PE | 2015 | Brazil/ | PE | 77/266 |
24.5 (24.8–28)/ | 38.7 (38.4–39)/ | Y | 8 | 1 | 11 | 60 | 3 | 43 | 217 | 13 | 131 | 49 | 477 |
| Caucasoid | 26.4 (25.3–28) | 35.2 (34.4–36) | ||||||||||||||||
| Rahimi-PE | 2013 | Iran/ | PE | 160/112 | NA/27.3 |
NA | Y | 8 | 0 | 38 | 122 | 4 | 14 | 94 | 38 | 282 | 22 | 202 |
| Caucasoid | ||||||||||||||||||
| Luizon-PE | 2012 | Brazil/ | PE | 122/102 | 27.4 |
36.2 |
Y | 8 | 1 | 29 | 92 | 2 | 14 | 86 | 31 | 213 | 18 | 186 |
| Caucasoid | 25.5 |
39.7 | ||||||||||||||||
| Luizon-GH | 2012 | Brazil/ | GH | 107/102 | 26.6 |
39.1 |
Y | 8 | 1 | 34 | 72 | 2 | 14 | 86 | 36 | 178 | 18 | 186 |
| Caucasoid | 25.5 |
39.7 | ||||||||||||||||
| Palei-GH | 2012 | Brazil/ | GH | 185/214 | 27.0 |
38.8 |
Y | 7 | 3 | 54 | 128 | 4 | 34 | 176 | 60 | 310 | 42 | 386 |
| Caucasoid | 24.5 |
39.8 | ||||||||||||||||
| Palei-PE | 2012 | Brazil/ | PE | 214/214 | 26.0 |
36.0 |
Y | 7 | 3 | 44 | 167 | 4 | 34 | 176 | 50 | 378 | 42 | 386 |
| Caucasoid | 24.5 |
39.8 | ||||||||||||||||
| Liu-HDP | 2009 | China/ | HDP | 71/66 | 27.9 |
36.7 |
N | 7 | 2 | 18 | 51 | 3 | 24 | 39 | 22 | 120 | 30 | 102 |
| Asian | 28.1 |
37.2 | ||||||||||||||||
| Fraser-PE | 2008 | UK/ | PE | 117/146 | 29 (16–42)/ | 35.6 (25–42)/ | Y | 8 | 1 | 34 | 82 | 4 | 28 | 114 | 36 | 198 | 36 | 256 |
| Caucasoid | 30 (16–40) | 39.5 (37–42) | ||||||||||||||||
| Coolman-PE | 2007 | Netherlands/ | PE | 163/163 |
28.0 |
34.6 |
Y | 6 | 1 | 16 | 128 | 2 | 31 | 118 | 17 | 273 | 35 | 267 |
| Caucasoid | 28.0 |
39.4 | ||||||||||||||||
| Note: HDP, hypertensive disorders of pregnancy; GH, gestational hypertension;
PE, preeclampsia; MMP, matrix metalloproteinases; HWE, Hardy-Weinberg
equilibrium; NOS, Newcastle-Ottawa Scale; n, number; Y, yes; N, no; a, the number
of cases and controls are not coincident
with the genotypes and/or alleles within the MMP9-1562 C/T due to the missing
data; b, data collected by mean | ||||||||||||||||||
We pooled data gleaned from the 10 reports (involving PE, GH,
and HDP) to evaluate the HDP risk associated with the MMP9
gene promoter region -1562 C/T polymorphism. For
the
genotypes (TT + TC vs CC)
and alleles (T vs C), the heterogeneity analysis of included in
10 reports indicates that the difference was statistically significant (Q =
26.32, 22.95; P = 0.002, 0.006; I
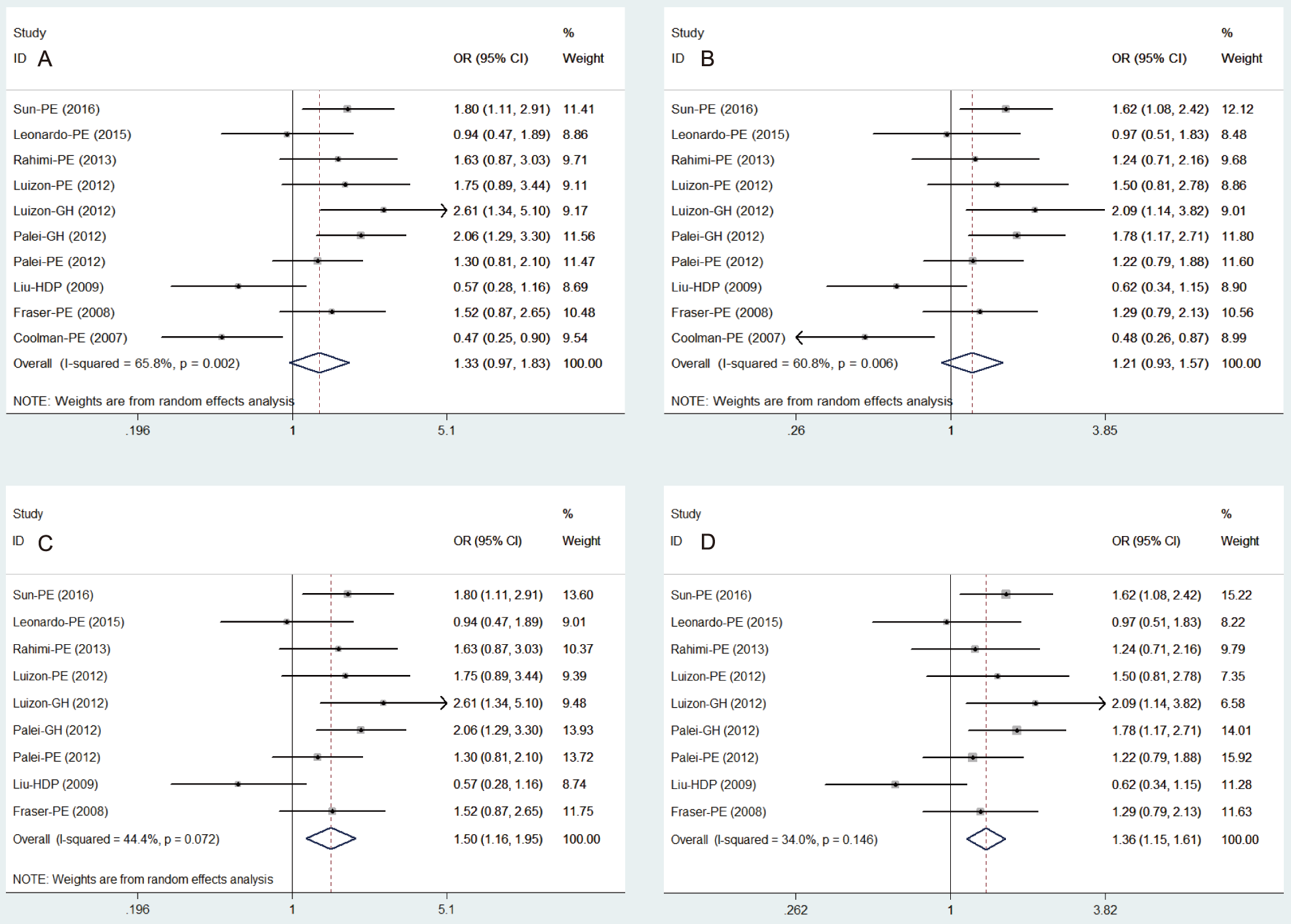 Fig. 2.
Fig. 2.Forest plot of the association between MMP9-1562 C/T polymorphism and HDP risk. (A,B) Pooled risk of HDP with the genotypes (TT + TC vs CC) and alleles (T vs C) of MMP9-1562 C/T. (C,D) Pooled risk of HDP with the genotypes (TT + TC vs CC) and alleles (T vs C) of MMP9-1562 C/T when one study (Coolman et al., 2007) [26] was excluded. HDP, hypertensive disorders of pregnancy; GH, gestational hypertension; PE, preeclampsia; MMP, matrix metalloproteinases.
Further research is necessary to reduce the heterogeneity of pooled analysis.
According to sensitivity analysis, the result showed that the study published by
Coolman et al. in 2007 [26] has great influence on the stability of the
model. Also, the study has been scored six and considered moderate quality study
by NOS. Therefore, the
study was eliminated from the meta-analysis. The result showed
a significant association between the genotypes of
MMP9-1562 C/T polymorphism
(TT + TC vs CC) and the susceptibility of HDP in a pooled group
of 9 reports (OR = 1.50, 95% CI 1.16–1.95, P = 0.002;
Fig. 2C) with the
random-effect model (Q =
14.38, P = 0.072, I
In view of the heterogeneity in the pooled analysis, we performed a further
subgroup analysis according to the classification diagnosis of
HDP. According to the classification diagnosis of HDP, a total
of 7 reports were included to analyse the relationship between
PE susceptibility and MMP9-1562 C/T genotype and allele. The
pooled ORs were found to be 1.27 (95% CI 0.91–1.77, P = 0.165;
Fig. 3A) and 1.15
(95% CI 0.87–1.53, P = 0.326;
Fig. 3B) with the random-effects model for each group in 7 studies
(Q = 13.73, 12.26; P = 0.033, 0.057; I
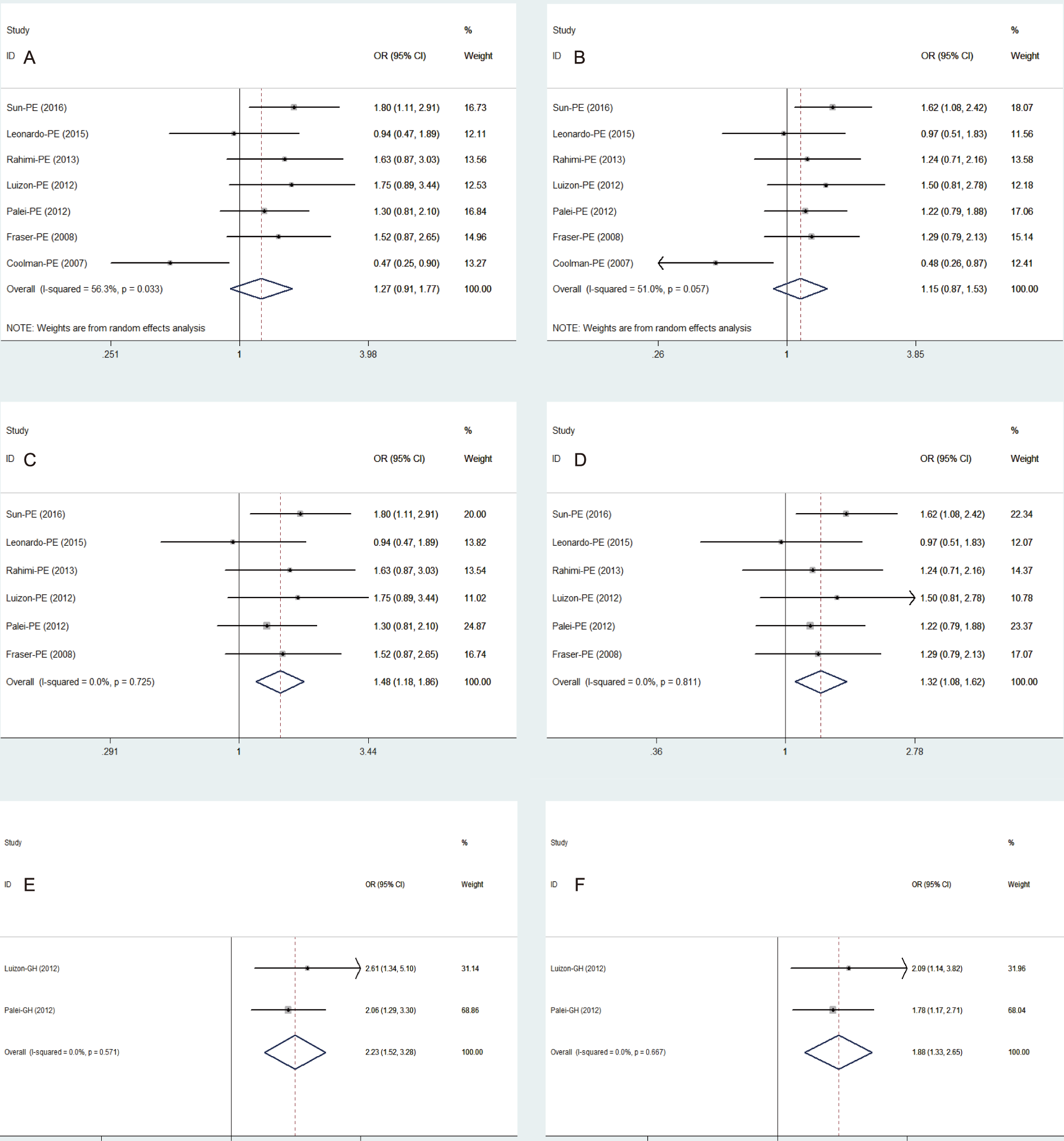 Fig. 3.
Fig. 3.Forest plot of the association between MMP9-1562 C/T polymorphism and PE/ GH risk. (A,B) Pooled risk of PE with the genotypes (TT + TC vs CC) and alleles (T vs C) of MMP9-1562 C/T. (C,D) Pooled risk of PE with the genotypes (TT + TC vs CC) and alleles (T vs C) of MMP9-1562 C/T when Coolman et al. (2007) [26] was excluded. (E) Pooled risk of GH with the genotypes (TT + TC vs CC) of MMP9-1562 C/T. (F) Pooled risk of GH with the alleles (T vs C) of MMP9-1562 C/T; PE, preeclampsia; GH, gestational hypertension; MMP, matrix metalloproteinases.
According to the classification diagnosis of HDP, a total of 2 reports were
included in the relationship between GH susceptibility and MMP9-1562 C/T genotype
and allele. The heterogeneity analysis of these two subgroups showed that there
was also no heterogeneity among the included studies (Q = 0.32, 0.18; P = 0.571,
0.667, I
In all the pooled groups mentioned above, neither the Begg’s (P
HDP is a pregnancy complication which manifests as multiple clinical subtypes. Many previous studies have reported the relationship between -1562 C/T polymorphism of MMP9 and HDP susceptibility, but disagreements between these studies were noted. One study indicated that pregnant women carrying MMP9-1562 TT + TC genotype are 2.37 times at increased risk of developing PE compared with control pregnant women [15]. Another study did not find a correlation between these two variables (OR = 0.304, 95% CI 0.045–2.065) [27]. In addition, work from the Luizon and Palei groups did not find the same risk between the -1562 C/T polymorphism of MMP9 and PE, respectively [6, 14]. However, both studies found that the MMP9-1562 C/T polymorphism not only increased the susceptibility of GH but also influenced the response of antihypertensive therapy [6, 14]. Two meta-analyses which included six publications were performed in 2014 and 2015 to evaluate the association between MMP9 gene -1562 C/T polymorphisms and the susceptibility to PE [16, 17]. However, the susceptibility to GH was not included in the published meta-analyses [18, 19].
Our group performed this pooled analysis to assess the relationship between MMP9-1562 C/T polymorphism and susceptibility of HDP including its subtypes. The preliminary results showed that the genotypes (TT + TC vs CC) and alleles (T vs C) did not increase the risk of the susceptibility to HDP with significant heterogeneity among the studies when data from all 10 reports were pooled together. When the study published by Coolman et al. [26], which had both a moderate-quality score and the greatest impact to the quantitative synthesis in sensitivity analysis, was eliminated, the results of meta-analysis revealed that the variant genotype (TT + TC) and allele (T) of MMP9-1562 C/T have the risk of 1.5 and 1.36 times in developing to HDP when compared with control pregnant women.
Previously, two meta-analyses including six publications in 2014 and 2015 revealed uniformly no association between MMP9-1562 C/T polymorphism and PE risk [16, 17].
Our preliminary subgroup analyses based on 7 studies also found no increased risk of PE from MMP9-1562 C/T polymorphism with the heterogeneity analysis having statistically significant. However, following the exclusion of the Coolman et al. [26] study, the heterogeneity among the pooled 6 reports decreased to 0.0% and the quantitative synthesis showed that the variant genotype (TT + TC) and allele (T) of MMP9-1562 C/T increased the risk of PE by 1.48 and 1.32 times, respectively. There are some tangible reasons for the different PE risk reported in this study and previous meta-analyses. First, one study searched the supplementary CNKI database, and two new studies published recently (2014 and 2015) have not been included in the previous meta-analyses [18, 19, 28]. Second, previous meta-analyses included two studies published by Palei et al. (2010 and 2012) which was considered as duplicate data in our study [14, 29]. Third, further subgroup analysis has been performed to decrease the heterogeneity among the pooled reports in the present meta-analysis. Concerning the subgroup analyses with no heterogeneity detected, the variant genotype (TT + TC) and allele (T) of MMP9-1562 C/T had a higher risk of GH susceptibility when compared with the control pregnant women (OR = 2.23 and 1.89). Thus, it can be concluded that MMP9-1562 (T) may be a potential genetic marker associated with HDP (GH and PE) which can help identify a susceptible population.
This study showed that -1562 C/T polymorphism in MMP9 gene promoter region can raise the HDP susceptibility, especially in GH and PE patients. Although the mechanism of this phenomenon is unclear, previous studies have suggested that with the presence of T allele in MMP9-1562 C/T the gene has a higher transcriptional activity and can express higher levels of MMP9 protein in tissues [30]. A large increase in MMP9 expression combined with the action of oxidative stress and inflammatory mediators may participate in the development of PE by promoting vascular endothelial dysfunction [31, 32]. In addition, high level expression of MMP9 protein may also play an important role in the processes of trophoblast invasion, placenta and uterine artery remodeling, and the regulation of vascular tension [13, 33]. Therefore, it is worthwhile in future research to clarify the concrete mechanism of this disease by exploring phenotype/genotype relationships of SNPs within the MMP9 gene promoter region.
This meta-analysis also has several limitations. First, due to the quantity restriction of the original studies, ethnicity subgrouping has not been performed. Second, HDP risk analyses with MMP9-1562 C/T polymorphism showed no patients with eclampsia, superimposed pre-eclampsia, and chronic hypertension in pregnancy were involved. Third, the subgroup analysis of GH susceptibility only involved two studies, so caution should be exercised when interpreting these results obtained with a small sample size. At last, in order to clarify the relationship between genetic polymorphisms in MMP9-1562 C/T and the risk of hypertensive disorders of pregnancy, it is necessary to balance the confounding factors with a larger sample size or conduct further mechanic research.
Overall, this meta-analysis revealed that the variant genotype (TT + TC) and allele (T) of MMP9-1562 C/T are associated with the susceptibility of HDP, especially with susceptibility for PE and GH. Further research with more convincing evidence for the association between MMP9-1562 C/T polymorphisms and the risk of the clinical subtypes of HDP such as GH and the functional consequences of MMP9 polymorphism is necessary.
JZ and YZ designed the research study. YZ and LW performed data extraction. RW and LW performed quality review. JZ and SC analyzed the data. YZ and JZ wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
We are thankful to all the authors and participants of the original studies included.
This study was supported by the Natural Science Foundation of China (No. 81803932), the Natural Science Foundation of Guangdong Province (Nos. 2018030310025 and 2017A030313868), and the President Foundation of Nanfang Hospital, Southern Medical University (Nos. 2017Z020 and 2016C024). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare no conflict of interest.
