1. Introduction
Uterine contractions are usually strengthened by oxytocin, prostaglandin
E2 (PGE2) and prostaglandin F2 (PGF2) through their actions
on oxytocin receptors (OXTR), PGE2 receptors (EP) and PGF2 receptors
(FP), respectively [1, 2, 3]. At term, PGE2 mediates myometrial contraction mainly
through the EP3 receptor subtype [4]. Generally recommended second-line agents
for the treatment of uterine atony are misoprostol, a known EP3/EP2 agonist [2],
and carboprost, which is a synthetic analog of PGF2 [5].
Postpartum hemorrhage (PPH) has an incidence of 4% and contributes to
nearly one quarter of all maternal deaths worldwide [6], most of which occur due
to uterine atony [7]. Oxytocin-induced or augmented labor contributes to uterine
atony by causing desensitization of the myometrium to oxytocin [8, 9]. Currently,
the treatment of uterine atony caused by oxytocin exposure is empirical [10, 11].
Therefore, the aim of the present study was to investigate changes in the
receptors involved in myometrial contraction following OXTR desensitization, thus
providing an experimental basis for the treatment of uterine atony.
Previous studies showed that OXTR expression was unable to increase at
term in mice due to lack of the FP gene [12, 13]. Subsequent studies showed that
treatment with PGF2 during pregnancy increased OXTR expression in human
myometrial cells from the lower uterine segment and decreased it in the upper
segment [14]. A recent study suggested that OXTR is an upstream regulator of
cyclooxygenase-2, which facilitates the conversion of arachidonic acid to PGE2
and PGF2 [14]. OXTR antagonists inhibit PGF2-induced
contractions and inflammatory responses in the human myometrium [15]. These
findings suggest that OXTR and FP expression and function may interact directly
or indirectly in the perinatal uterus.
We hypothesized that desensitization of OXTR would result in
compensatory increases in the expression and activation of FP and EP3. We also
hypothesized that the effects of prostaglandins and oxytocin on OXTR expression
might be different in myometrium with OXTR desensitization. The primary goal of
this in vitro study was therefore to explore changes in EP3, FP and OXTR
expression in late-pregnancy rat myometrium following pretreatment with oxytocin,
as well as their activation effects. The second goal was to investigate the
effect of prostaglandins on OXTR expression.
2. Methods
2.1 Experimental animals
All animal experiments were approved by the Animal Ethics Committee of
the Shanghai Medical College, Fudan University [Approval number: 201907007Z].
Pregnant Sprague-Dawley rats (age 12 weeks, weight 280 to 350 g, 16 days of
gestation as determined by the presence of a copulatory plug at gestational day 0
or 1) were purchased from Shanghai Jiesijie Experimental Animal Co. Ltd. (No.
1068, Zhaotai Road, Minhang District, Shanghai, China). The rats were housed for four
days in the animal facility and maintained on ad libitum standard rat
chow and tap water in a 12:12 hour light-dark cycle. The temperature of the
breeding room was kept at 20~25 C and the relative
humidity at 50~65%.
2.2 Myometrial strip isolation and preparation
Rats were euthanized by injecting excess pentobarbital (140 mg/kg)
through the right groin and into the abdominal cavity. After carefully removing
the contents of the uterine cavity, the remaining uterine smooth muscle was
placed in physiological salt solution (PSS: 120 mM NaCl, 5.9 mM KCl, 25 mM
NaHCO, 1.2 mM NaHPO, 11.5 mM dextrose, 2.5 mM CaCl, 1.2
mM MgCl) precooled to 4 C. The adipose and vascular tissues
surrounding the uterine tissue were carefully separated and removed under the
microscope. For the isometric contraction experiment, the uterine tissue was cut
into 7-mm-long and 3-mm-wide strips along the longitudinal axis of the muscle
[15]. Three or four myometrial strips were obtained from each rat. Longitudinal
myometrial strips were vertically suspended in a thermostatic water bath at 37
C with 35-mL liquid at pH 7.4. Gas (95% oxygen and 5% carbon dioxide)
was pumped into the liquid continuously [16]. Myometrial contractions were
continuously recorded using an isometric force transducer connected to a
4-channel physiological signal acquisition and processing system (Jide
Experimental Instrument Factory, Shanghai, China). The myometrial contraction
record was analyzed using the RM6240 series multi-channel physiological signal
acquisition and processing system. Each myometrial strip was equilibrated in PSS
at 1-g tension for 40 min to achieve regular contraction and to adapt to the
environment. When the myometrium reached a regular contraction pattern, each
myometrial strip was stimulated with 96-mM KCl to induce a contraction that
reflected the maximum contractile capacity of the tissue [16]. The KCl solution
was then drained from the organ bath and residual solution was removed by washing
three times with PSS [16]. The prepared myometrial strips were used for
subsequent experiments. The flow diagram for the experiments is shown in Fig. 1.
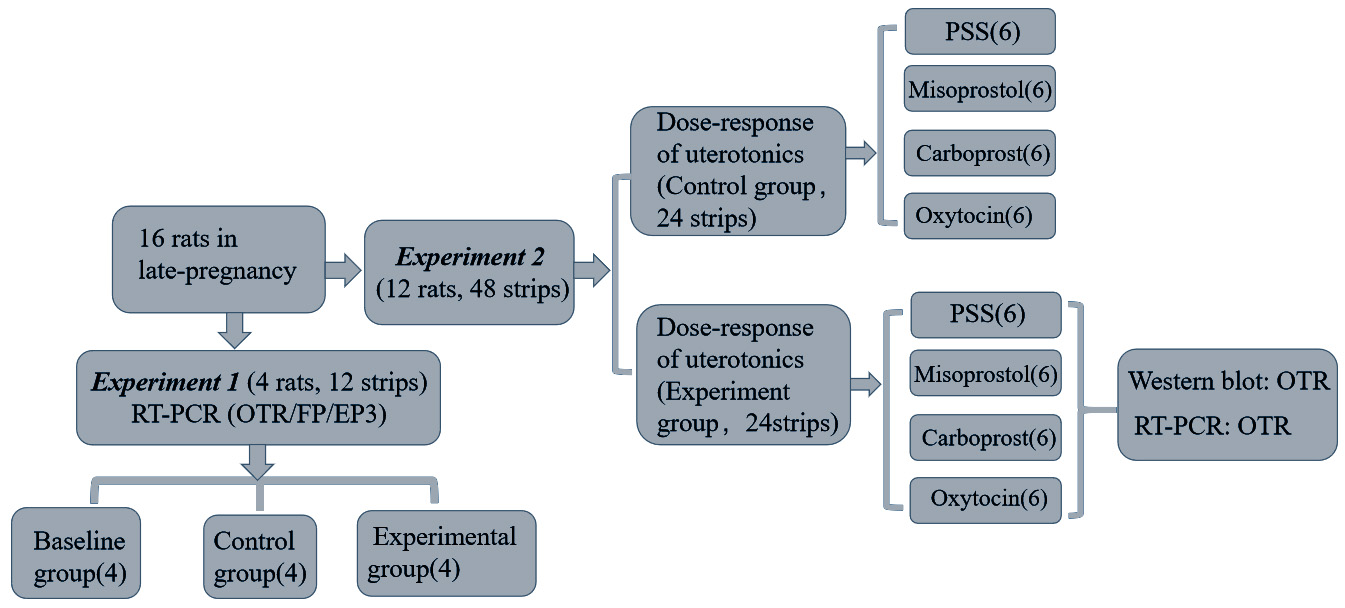 Fig. 1.
Fig. 1.
Flow diagram of the study. A total of 16 late-pregnancy
rats were used. In Experiment 1, 3 myometrial strips were
isolated from each of 4 rats and randomly distributed to baseline, control
(equilibration in PSS for 2 hrs) and experimental groups (treatment with 10 M oxytocin for 2 hrs). In Experiment 2, 4 myometrial strips
were isolated from each of 12 rats in order to test the dose-response of oxytocin
(10 to 10 M), misoprostol (10 to 10 M), carboprost
(10 to 10 M) and PSS. Before the dose-response testing, two
myometrial strips were equilibrated in PSS for 2 hrs (control group) and the
other two were treated with 10 M oxytocin for 2 hrs (experimental group).
The control group and the experimental group for the same drug were evaluated
simultaneously, and the uterine smooth muscle isolated from the same rat. The
number in each box reflects the sample size.
3. Calculation of contractile activity
The contractile activity of the myometrium was calculated as the average
tension (g) frequency (contractions/15 min). The ratio of
contractility was used for statistical analysis and was calculated as the
contractile activity at each drug concentration divided by the spontaneous
activity. The spontaneous activity of each myometrium strip was calculated during
15 min of stable contractions prior to the addition of 10 M oxytocin/PSS.
4. Experiment 1: mRNA expression for EP3, FP
and OXTR
4.1 Inducing the desensitization of OXTR
Experiment 1: three myometrial strips were obtained from each of four
rats and randomly distributed to the baseline, control and experimental groups.
The baseline group was used to extract total RNA just after
muscle strip preparation. In the experimental group, myometrial strips were
pretreated with 10 M oxytocin for 15 minutes and then with 10 M
oxytocin for 2 hours to induce OXTR desensitization. Myometrium strips were then
rested in fresh PSS for 10 min to adapt to the subsequent lower concentration of
10 M oxytocin (Fig. 2A) [17]. The myometrium contractile activity in
10 M oxytocin was assessed before and after the induction of OXTR
desensitization and the values were compared. The control group was exposed to
PSS rather than 10 M oxytocin for 2 hours, and the contractile responses
compared to those observed in the experimental group. The myometrial strips were
collected at the end of the contraction experiment. Reverse
transcription-polymerase chain reaction (RT-PCR) assays were used to evaluate the
mRNA expression of EP3, FP and OXTR in the baseline, experimental and control
groups.
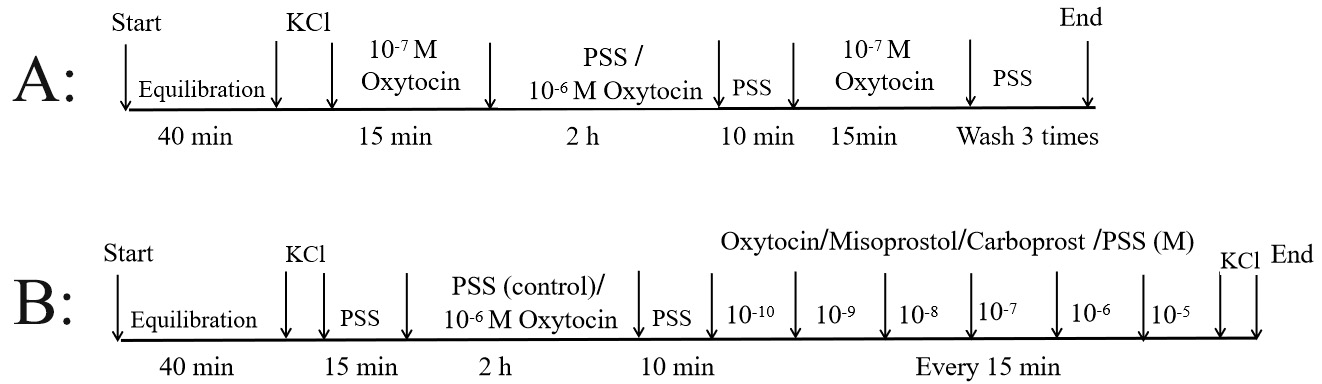 Fig. 2.
Fig. 2.
Experimental evaluation of myometrial
contraction. Experiment 1: following treatment with PSS or 10 M oxytocin
(to induce oxytocin receptor desensitization) for 2 hrs, myometrial strips were
washed 3 times with PSS and assessed for mRNA expression of OXTR, EP3 and FP
receptors (A). Experiment 2: following treatment with PSS (control group) or
10 M oxytocin (experimental group) for 2 hrs, myometrial strips were
exposed to different uterotonic agents. Only myometrium samples from the
experimental group were collected for evaluation of oxytocin receptor expression
(B). KCl, potassium chloride; PSS, physiological salt solution.
4.2 Real-time RT-PCR
Total RNA was extracted using TRIzol reagent (Life Technologies, 21-22F,
L’Avenue, 99 Xianxia Road, Changning District, Shanghai, China) and cDNA was synthesized
using the ReverTra Ace qPCR RT Kit (FSQ-101; TOYOBO, Osaka, Japan) according to the
manufacturer’s instructions. Quantitative RT-PCR was carried out using
SYBR Green Real-time PCR master mix (TOYOBO) with a
CFX96 Real-Time System instrument (BIO-RAD, Hercules, CA, USA). Each reaction was
run in triplicate to minimize variation. Gene expression was normalized to the
mean expression of the housekeeping gene GAPDH. The experiment was repeated four
times. The PCR primers were as follows: GAPDH forward primer
5-TGCACCACCAACTGCTTAGC-3anreverse primer
5-GGCATGGACTGTGGTCATGAG-3; OXTR forward primer
5-TAGGTGATGGCGTATGTTTGTG-3and reverse primer
5-GTTGTCTGATGGCTGAGTCCC-3; EP3 forward primer
5-ACTGTCCGTCTGCTGGTC-3and reverse primer
5-CCTTCTCCTTTCCCATCTG-3; FP forward primer
5-GAGATTTAGACGGAAGTCGAAGG-3 and reverse primer
5-GTGATCACCAGGCCACTAGC-3.
5. Experiment 2: effect of uterotonic agents
on OXTR expression
5.1 Contractility analysis
Experiment 2: following treatment with PSS (control group) or 10
M oxytocin (experimental group) for 2 hours, the myometrial strips were subjected
to dose-response testing with oxytocin (10 to 10 M), misoprostol
(10 to 10 M), carboprost (10 to 10 M) or PSS (Fig. 2B) [18, 19, 20]. For each drug the control and
experimental group tests were conducted simultaneously and the myometrium samples
were obtained from the same rat. According to a previous study, a concentration
of 10 M oxytocin produces the maximum contraction in isolated rat
myometrium [16]. In our preliminary experiments, 10 M oxytocin induced
tetanic contraction of the myometrium with high mean tension. Therefore, the
maximum concentration of oxytocin used in the present study was 10 M.
After finishing the uterotonic-stimulated contractions, each myometrial strip was
stimulated with 96-mM KCl to evaluate its activity. Following completion of the
contraction experiment, myometrial strips from the experimental groups
were divided into two halves to evaluate the expression of OXTR. One of the
halves was used for Western blot analysis and the other for RT-PCR.
5.2 Expression of OXTR mRNA
OXTR mRNA expression was evaluated using the same method described for
real-time RT-PCR.
5.3 Expression of OXTR proteins
Western blot analysis: Tissue proteins were extracted using a
radioimmune precipitation assay RIPA lysis buffer (P0013B, Beyotime, Jiangsu,
China) and separated by 10% SDS-PAGE. The detached protein was transferred onto
a polyvinylidene difluoride (PVDF) membrane. The membrane was then blocked with
5% skim milk dissolved in PBST (PBST: KCl, 2.68 mM; KHPO, 1.47 mM;
NaCl, 136.89 mM; NaHPO.12HO, 8.06 mM; 0.1% TWEEN-20.) for 2
hours at room temperature to reduce nonspecific background. PVDF membranes were
incubated at 4 C overnight with primary antibodies to oxytocin receptor (1 :
5000; ab181077 supplied by abcam) and GAPDH (1 : 10,000; ab181602 supplied by
abcam). Each membrane was then incubated with a secondary antibody (1 : 5000;
7074P2; CST) for 2 hours at room temperature. Finally, the membranes were treated
with enhanced chemiluminescence (Bio-Rad) and observed using a Western blot
visualizer (Tanon 5500; Tanon, Shanghai, China). The experiment was repeated four times
and the intensity of bands was quantified by Image J.
6. Statistical analysis
All data are expressed as mean standard deviation (SD).
The concentration-response curve was obtained by taking drug
concentrations as the abscissa and ratio of contractility as the ordinate
variable. The curve was fitted using Prism 8 (GraphPad Prism Software, San Diego,
CA, USA) . The Student’s t-test was used to compare differences between
the control and experimental groups for each uterotonic agent. The potency pEC50
(negative logarithm of the molar concentration required to elicit 50% maximum
contraction response) and efficacy (maximum response [Emax(ratio)]) of each drug
were compared between groups with one-way analysis of variance (ANOVA) and
Dunnett’s post hoc (2-sided) test using SPSS Statistics 22 software (IBM, Armonk,
NY, USA). In all cases, differences were considered significant at a P
value of 0.05.
7. Results
Myometrial contractile amplitude and frequency showed no significant
changes during continuous exposure to PSS for 2 hours (Fig. 3A1). The response
to 10 M oxytocin before and after this process also showed no difference.
However, the baseline level, amplitude, and frequency of myometrial contraction
gradually decreased during 2 hours of 10 M oxytocin treatment (Fig. 3A2).
The contraction response of myometrium to 10 M oxytocin was significantly
weaker than prior to the 10 M oxytocin pretreatment (P = 0.005)
(Fig. 3B). The mRNA expression levels of EP3, FP and OXTR did not change
significantly with and without oxytocin
pretreatment (Fig. 3C1–C3).
 Fig. 3.
Fig. 3.
Experiment 1: Receptor mRNA expression in
myometrium from the experimental group (with oxytocin pretreatment) and from the
control group (without oxytocin pretreatment). (A) Representative isometric
tension recordings for the control group (A1) and for the experimental group
(A2). The time scale was suitable for Fig. A1 and Fig. A2. (B) Myometrial
contractile activity before and after desensitization with 10 M oxytocin
or PSS for 2 hrs of contraction, stimulated by 10 M oxytocin. #
statistically significant, P = 0.005 (paired-samples T test).
(C) mRNA expression levels for the PGE2 receptor (EP3; C1), PGF2
receptor (FP; C2), and oxytocin receptor (OXTR; C3) were measured and normalized
to the housekeeping gene GAPDH. No statistical differences in the mRNA levels of
these receptors were observed between the control and experiment groups. Data are
mean SD (whiskers). OT, oxytocin; PSS, physiological salt solution.
Without oxytocin pretreatment, the concentration-response curve for
misoprostol was flat. However, with oxytocin pretreatment the myometrial
contractility increased rapidly between misoprostol concentrations of 10
to 10 M (Fig. 4). Misoprostol had a more obvious contractile effect on the
myometrium after oxytocin pretreatment compared to the control (mean (SD) Emax(ratio): 4.44 1.47 vs 1.32 0.22, P = 0.02).
There was no significant difference in the maximum myometrial contraction effect
produced by carboprost with or without oxytocin pretreatment. However, carboprost
significantly enhanced the contractile potency of myometrium pretreated with
oxytocin compared to the control (pEC50: 7.74 0.56 vs 6.81
0.25, P = 0.03). At any oxytocin concentration, myometrial
contractility induced by oxytocin in the experimental group was significantly
lower than that observed in the control group (Emax(ratio): 1.62 0.27
vs 2.82 0.98, P = 0.015). Myometrial contractile
activity in the control group was greater than that of the oxytocin pretreatment
group at any time in PSS (Table 1).
Table 1.Myometrial contractile activity to uterotonics in rat isolated
myometrial strips with or without oxytocin pretreatment.
|
Oxytocin |
Misoprostol |
Carboprost |
| Control group |
|
|
|
| n1 |
6 |
6 |
6 |
| Emax(ratio) |
2.82 0.98 |
1.32 0.22 |
2.74 0.44 |
| pEC50 |
9.53 0.37 |
10.23 0.25 |
6.81 0.25 |
| Experiment group |
|
|
|
| n2 |
6 |
6 |
6 |
| Emax(ratio) |
1.62 0.27 |
4.44 3.60 |
2.40 1.25 |
| pEC50 |
9.00 0.51 |
8.27 0.29 |
7.74 0.56 |
| P1 value |
0.02 |
0.02 |
0.23 |
| P2 value |
0.11 |
0.00 |
0.03 |
| The Emax(ratio) and pEC50 were compared among three uterotonics in each
group (“a” means P 0.02, one-way analysis of variance, Dunnett’s
post hoc comparison to oxytocin). Emax(ratio) = the maximum myometrial
contractility of responding to uterotonics divided by baseline contractile
activity; pEC50 = negative logarithm of the concentration of uterotonic agent
required to elicit 50% maximum response. P1 value: compare the
difference of Emax(ratio) between myometrial strips with and without oxytocin
pretreatment to the same drug. P2 value: compare the difference
of pEC50 between myometrial strips with and without oxytocin pretreatment to the
same drug. “b” means P1 value and P2 value
0.05, which were accepted as statistically significant. Data represent mean
SD; n = number of myometrial strips from separate rats. |
In the control group, the maximum contractile effect of oxytocin on
myometrium was significantly greater than for misoprostol, but was not
significantly different to that of carboprost. However, the contractile potency
(pEC50) of oxytocin on myometrium was significantly greater than that of
carboprost. In the experimental group, the potency of oxytocin on myometrial
contraction was significantly greater than that of both misoprostol and
carboprost. However, there was no significant difference in the maximum
contractile effect between the three groups.
After completing the contraction tests stimulated by uterotonics, the
myometrial strips for the experimental group were evaluated for OXTR expression
at the mRNA and protein levels. Compared to continuous oxytocin exposure, OXTR
expression increased significantly following equilibration in PSS. There was no
significant difference in OXTR expression in myometrium between the misoprostol,
carboprost and oxytocin treatment groups. Moreover, there was no statistical
difference in OXTR expression between the PSS treatment and misoprostol or
carboprost treatment groups (Fig. 5).
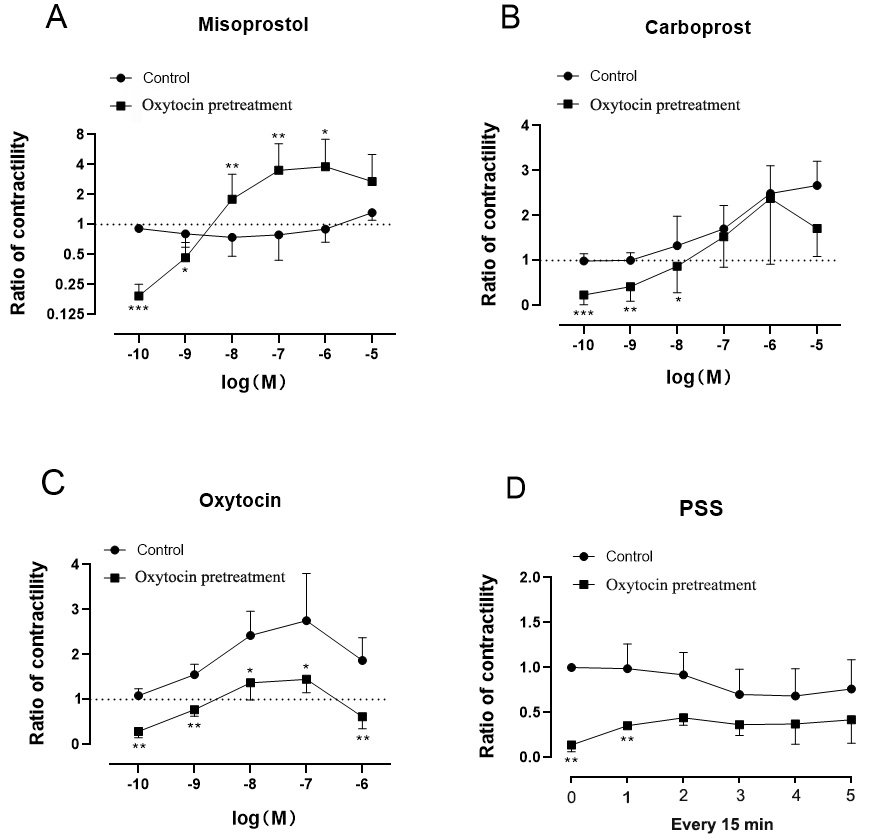 Fig. 4.
Fig. 4.
Experiment 2.
Concentration-response curves for misoprostol (A), carboprost (B), oxytocin (C)
and PSS (D) in late-pregnancy rat myometrium, with or without oxytocin
pretreatment. Contractile responses are shown as ratios of contractility. The
ratio of contractility was calculated as the contractile activity at each drug
concentration divided by the baseline spontaneous activity. Data are mean
SD (whiskers). The difference in ratio between the control group (n = 6) and
oxytocin pretreatment group (n = 6) was compared using T-test at each
drug concentration (at each period in the PSS group). “*” represents 0.01 P 0.05, “**” represents 0.001 P 0.01, “***”
represents P 0.001.
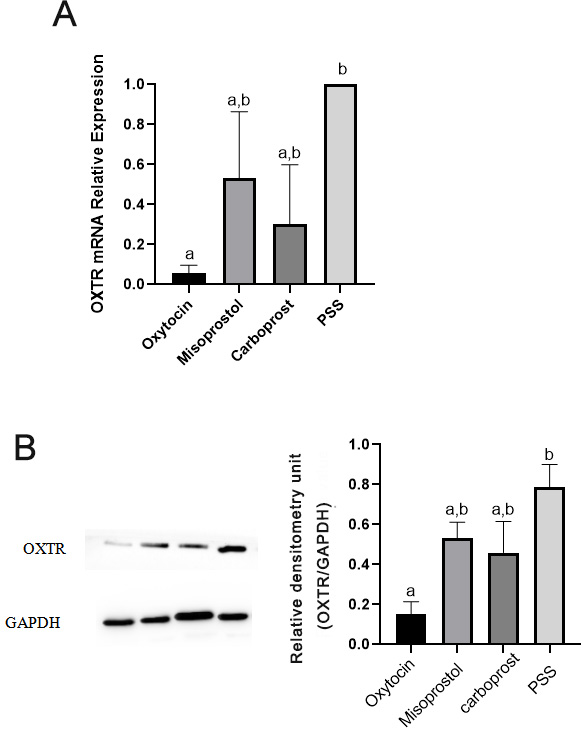 Fig. 5.
Fig. 5.
Experiment 2. Oxytocin receptor (OXTR)
expression. Expression of the housekeeping gene GAPDH was used as an internal
control to assess the expression of OXTR mRNA following treatment of myometrium
with oxytocin, misoprostol, or carboprost (A). Whole-tissue lysates were
subjected to Western blotting. GAPDH served as a loading control, and blots were
scanned for densitometric analysis (B). Representative Western blots are also
shown. Data are mean SD (whiskers). Significant differences (A,B)
between groups are illustrated by different lowercase letters above each bar;
groups sharing the same letter did not differ (one-way analysis of variance,
Bonferroni post hoc comparison). PSS, physiological salt solution.
8. Discussion
Following pretreatment in vitro with 10 M oxytocin for 2
hours, the contractile response of myometrium to oxytocin decreased
significantly, although mRNA expression for OXTR, FP and EP3 did not change.
After oxytocin pretreatment, the sensitivity of myometrium to carboprost
increased, but the maximal contraction induced by carboprost did not change.
Misoprostol had an obvious contractile effect on myometrium pretreated with
oxytocin, but was inactive for myometrial contraction in the control group.
Following the desensitization of OXTR, continuous oxytocin exposure reduced OXTR
expression compared to equilibrium in PSS. Furthermore, there were no
statistically significant differences between the PSS, carboprost and misoprostol
groups for OXTR mRNA expression.
Oxytocin acts directly on phosphoinositidase C-linked G protein coupled
receptors (OXTR) to increase cytosolic Ca to strengthen myometrial
contraction. Many literatures as well as our experimental results demonstrated
that oxytocin exposure would induce desensitization of OXTR. In the research of
Phaneuf et al. [21], oxytocin exposure decreased the binding of oxytocin to
cell membranes, however flow cytometry experiments demonstrated that OXTR were
not internalized during this treatment. The second messengers calcium,
inositol phosphates (InsPs) and cyclic nucleotides play decisive roles in uterine
contractility [22]. In a previous study, oxytocin-induced desensitization did not
change the ability of PGF2 to increase intracellular free calcium, did
but change such ability for oxytocin [17]. The present study confirms that FP
expression was not affected by oxytocin pretreatment, and that the myometrial
contractile potency of carboprost became stronger. One possible explanation is
that oxytocin-induced desensitization acts only on OXTR levels without affecting
post-receptor signaling. Earlier studies involving human and rat myometrial
tissues also showed that pretreatment with oxytocin decreased the response to
subsequent oxytocin exposure, but the myometrium consistently responded to
PGF2 stimulation [17, 18].
As shown in Fig. 3, treatment with 10 M oxytocin for 2 hours
caused OXTR desensitization and the response to 10 M oxytocin before and
after this process showed significant differences (A2, B). However, the level of
OXTR mRNA did not change (C3). In the oxytocin pretreatment group, OXTR
expression decreased significantly following continuous exposure to oxytocin
compared with equilibration in PSS (Fig. 5). We speculate that continuous use of
oxytocin initially decreases the response of myometrium to oxytocin, while the
decreased expression of OXTR mRNA needs to further prolong the time of oxytocin
treatment. Although it is believed that oxytocin/OXTR signaling is essential,
numerous studies have shown that OXTR expression does not correlate with the
oxytocin-induced uterine contraction effect. A recent study showed that peaks in
oxytocin level during labor did not correlate with the time of uterine
contractions [23]. Another study showed that late-pregnancy uterine contractions
in mice are mainly controlled by modification of the contractile signal machinery
rather than by the level of OXTR [24]. The decrease in OXTR mRNA in myometrium
accompanied by the decline in response to oxytocin might be related to the longer
time of oxytocin pretreatment [21].
A previous study showed that EP3 receptor-deficient mice have normal
parturition [25]. Similarly, the present study found the EP3 agonist misoprostol
had no contractile effect on late-pregnancy rat myometrium in the control group.
However, we also found that misoprostol had a significant myometrial contractile
effect following oxytocin-pretreatment, even though EP3 mRNA did not increase.
Misoprostol is a mixed EP3/EP2 receptor agonist [26]. During human pregnancy,
myometrial EP3 receptors are excitatory while EP2 receptors are inhibitory [27].
Therefore, we speculate the myometrial contractile effect of misoprostol
following oxytocin-pretreatment may be due to inhibition of EP2 expression or
activity. In contrast to the present results, Balki et al. [28] showed that
responses to PGF 2 and to misoprostol were not affected by labour or by
prior exposure to oxytocin. A possible reason for the discordant results is
that the myometrium in their research was balanced in PSS for 2 hours before
administering uterotonics and was not exposed to oxytocin, thus causing the
myometrium to re-sensitize to oxytocin [29].
Following OXTR desensitization, both the contractile potency of
carboprost and the maximum contractility of misoprostol increased significantly.
This suggests it was reasonable and necessary to use FP and EP3 receptor agonists
to enhance myometrial contraction after OXTR desensitization. Following
pretreatment with oxytocin, OXTR expression was significantly higher in the PSS
group compared to the oxytocin exposure group, while OXTR expression in the
carboprost and misoprostol groups was not significantly different compared to the
PSS group. A previous study on human myometrium also demonstrated that oxytocin
combined with carboprost produced a better contractile effect compared to
oxytocin alone following oxytocin pretreatment [30]. Recent literature has also
reported that misoprostol plus oxytocin was a more effective strategy for
preventing PPH than oxytocin alone [10, 31]. Therefore, combinations or alternate
uses of oxytocin, FP and EP3 receptor agonists may be more effective at
strengthening myometrial contraction.
Balki et al. [30] reported there was incomplete information on the
plasma levels of uterotonics following parenteral administration in the setting
of PPH. They suggested that oxytocin levels vary significantly during
pregnancy, labor and postpartum from 10 to 10 M and may not
accurately reflect the local myometrial concentration [32, 33, 34, 35]. Because the
release of oxytocin during physiological labor is pulsatile, the measured values
of serum oxytocin concentration vary greatly depending on the sampling intervals.
Oxytocin levels doubled in response to a doubling of the infusion rate of
exogenous oxytocin [23]. Thus, accurate plasma levels of oxytocin are difficult
to define. The approximate peak serum level of carboprost after intramuscular
injection of 250 g was approximately 10 M in term pregnant women
[36]. Misoprostol is commonly administered into the vagina and hence the plasma
level of this drug may differ significantly from the concentrations to which the
uterine smooth muscle is exposed.
Although the in vitro drug concentrations used in the present
study may not directly reflect serum levels in vivo, the concentration
range (10 to 10 M) investigated here may well include the
physiologic serum levels of these drugs. Morrison et al. [20] provided a
detailed pharmacodynamic analysis of uterotonics on isolated myometrium from
women undergoing elective cesarean delivery at term. Their results showed
similar pEC50 for oxytocin and carboprost to the present study, while misoprostol
was also inactive in myometrial contraction. Hence, the previous literature
suggests that rat and human myometrial tissues have similar pharmacodynamic
responses to uterotonics.
The present study of the receptor activation effect confirmed our
hypothesis that carboprost and misoprostol enhance myometrial contraction
following OXTR desensitization. However, we did not further explore the possible
mechanism of OXTR desensitization, such as the oxytocin-induced effect of
increased cytoplasmic free calcium concentration before and after receptor
desensitization. Pretreatment with oxytocin for 2 hours increased EP3 mRNA
expression, although this did not reach statistical significance. It is unclear
whether prolonging the duration of oxytocin exposure would lead to significantly
increased EP3 mRNA expression. Misoprostol also acts on EP2 to inhibit myometrial
contraction [27], but we did not further investigate the effect of oxytocin
pretreatment on EP2. In vitro experiments based on animal tissues have
inherent limitations, such as species variation and differences between
in vivo and in vitro environments.
In summary, we found that following pretreatment with 10 M
oxytocin for 2 hours, the response ability of myometrium to subsequent oxytocin
decreased significantly. However, the mRNA expression levels of EP3, FP and OXTR
were not affected by pretreatment with oxytocin, whereas the contractile potency
of carboprost and the maximal contractile effect of misoprostol both increased
significantly. No significant difference in OXTR expression was observed between
oxytocin and prostaglandin treatments. Misoprostol was inactive in normal
myometrial contraction. These experimental results suggest that prostaglandin
uterotonics, especially misoprostol, may have desirable therapeutic effects on
uterine atony caused by long-time oxytocin exposure. Further studies are required
to confirm this hypothesis.
Author contributions
LL and SH designed the research study. LL and JH performed the research.
TW analyzed the data. All authors contributed to editorial changes in the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All animal experiments were approved by the Animal Ethics Committee of
the Shanghai Medical College, Fudan University [Approval number: 201907007Z].
Acknowledgment
Thanks for the department of physiology & pathophysiology of Fudan
University Shanghai Medical College for providing laboratory. Thanks to the
researchers of this laboratory for their guidance on experimental technology.
Funding
This study was funded by the General Foundation of Shanghai Municipal
Health Commission (201840327).
Conflict of interest
The authors declare no conflict of interest.
 Fig. 1.
Fig. 1. Fig. 2.
Fig. 2. Fig. 3.
Fig. 3. Fig. 4.
Fig. 4. Fig. 5.
Fig. 5.