Academic Editor: Antonio Simone Laganà
Background: Problems with hormonal changes and the related
variations in bone turnover in adolescents with polycystic ovarian syndrome
(PCOS) have been of interest in terms of providing these patients with an
opportunity to receive a prophylactic and precision-based treatment aiming to
prevent early onset of osteoporosis. Materials and methods: Prospective comparative clinical trial—‘case-control’ type in Bulgarian
populace of 36 female patients with PCOS and 42 healthy controls aged 12 to 18.
The study protocol included a general section of anthropometric patient data,
clinical section–including general and Ob/Gyn Medical History, ultrasound exam
of the lesser pelvis and a lab section examining the serum levels of
Follicle-stimulating hormone (FSH), Luteinizing hormone (LH), estradiol,
Anti-Müllerian hormone (AMH) and bone turnover markers–osteocalcin and
PCOS is a heterogenic endocrine disorder that affects 1 in 15 women worldwide [1]. Usually the onset of the condition is during adolescence and the clinical presentation is characterized by anovulatory cycles (amenorrhea, opsomenorrhea, irregular menstrual cycles) combined with hyperandrogenism symptoms (hirsutism, acne, alopecia).
Due to the varied expressions of the syndrome, the European Society for Human Reproduction and Embryology (ESHRE) and the American Society for Reproductive Medicine (ASRM) have established that the diagnosis requires the presence of at least two of the “Rotterdam Criteria”.
- Polycystic ovaries on ultrasound.
- Anovulatory menstrual cycles.
- Clinical or biochemical evidence of excess androgen [2, 3].
One of the basic irregularities of this syndrome is the increased serum levels of LH and the imbalanced ratio of LH versus FSH. The paraclinical characteristic of PCOS patients is increased LH serum levels and normal FSH levels. It is considered to be due to increased release frequency of GnRH from the hypothalamus which leads to a hyperfunction of the theca cells [4]. The estradiol serum levels are similar to those of the healthy controls but still of lower values and they remain constant along the duration of the whole menstrual cycle. AMH is usually significantly higher in PCOS patients, regardless of obesity and evidence of excess androgen, although other studies have recorded no statistically significant variance between those patients and healthy controls [5, 6]. The high diagnostic reliability of AMH as a surrogate marker complementary to the Rotterdam Criteria in diagnosing PCOS patients has been confirmed in multiple trials [7, 8, 9, 10, 11, 12].
Most of the patients with PCOS display high BMI which prevents
amenorrhea/oligomenorrhea-induced bone loss. The adipocytes and the stromal cells
in the adipose tissue express P450 aromatase which helps adrenal and ovarian
testosterone and androstenedione convert to 17
Increase of BMI is associated with the increase of bone mineral density. It has
been reported that the risk of osteoporosis-related fractures is lower in
overweight women compared to those with normal or low values of BMI [13, 14].
Ravn et al. [15] compared women with obesitas to same age women with
normal weight and concluded that the former group had a significantly lower risk
of developing osteoporosis. On the other hand, obesity can cause bone loss by
stimulating the formation and excretion of proinflammatory cytokines-IL-6 and
tumor necrosis factor-
Bone tissue is metabolically active during the whole human life cycle. Monitoring bone metabolism is achieved by assessment of bone formation markers (alkaline phosphatase, osteocalcin, collagen propeptides) and bone resorption markers (beta C-terminal cross-linked telopeptide of type I collagen, bCTX). Bone markers are highest at birth and remain so during childhood, reflecting the most rapid bone growth and most intense acting processes of modelling and remodelling of the bones. Gradual reduction of marker levels follows that period and only during the puberty growth spirt (12–13 years of age) does a new upswing occur with the peak between 10 and 13 years of age in girls [18, 19].
Puberty is associated with accelerated bone metabolism [20, 21, 22]. After puberty follows a decline in bone marker levels (most pronounced between 30 and 50 years of age) and going into menopause marks an upswing in their values once more due to the direct effect of the menopause itself and the related hormonal deficit [23].
There is very controversial data when it comes to bone metabolism markers in PCOS patients as some authors report significantly lower values of bone formation markers and normal or lower levels of bone resorption markers. This data has not been confirmed by other studies conducted among adolescents with PCOS and where no significant variance has been noted compared to the control group in terms of bone metabolism and bone mineral density [24, 25, 26, 27, 28].
Vitamin D (Vitamin D - 1,25(OH)2 Vitamin D) plays a central role in bone formation and bone remodeling [29, 30, 31]. Multiple studies have asserted the importance of maintaining adequate Vitamin D serum levels to protect against bone fractures [32, 33, 34, 35, 36, 37]. There is a number of studies that have not recorded a significant variance in Vitamin D serum levels in PCOS patients and controls [38, 39, 40], however, others have reported significantly higher levels in PCOS patients compared to healthy individuals of the same age [41, 42]. Lagowska studied the Vitamin D serum levels of 77 patients with opso-(PCOS) and amenorrhea (primary and secondary) and of a control group consisting of women with normal menstrual cycle and discovered significantly lower values of Vitamin D in patients with hypomenstrual disorders compared to controls. The observed Vitamin D deficit in patients is associated with high levels of PTH, anovulation, hyperandrogenemia and infertility [43].
This prospective trial “case-control” type studied 42 healthy “controls” and 36 patients with PCOS aged 12 to 18 for the period 2015–2019 in University hospital “Maichin dom”, Sofia.
The PCOS patients included in the trial have met at least two of the three Rotterdam Criteria as established by ESHRE/ASRAM in 2003.
The control group included girls between 12 and 18 years of age with normal menstrual cycles, normal ultrasound findings–uterus and ovaries with no pathological deviations and normal or reference serum levels of the main lab indicators.
The exclusion criteria for both groups were: endocrine pathology with thyroid, adrenal origin; severe acute and chronic conditions; congenital conditions impacting the musculoskeletal system; primary amenorrhea due to Swyer syndrome, Morris syndrome, MRKH syndrome; use of oral contraceptives, corticosteroid drugs, hormonal drugs; pregnancy; refusal to participate in the trial.
The protocol of the trial included — general section of anthropometric patient
data–age, height, weight, BMI; clinical section — general and Ob/Gyn Medical
History, ultrasound exam, lab section-examining the serum levels of LH, FSH,
estradiol, AMH and osteocalcin,
Follicle-stimulating hormone (FSH — Reference Range Female, Follicular phase: FSH — 1–10 IU/L), Luteinizing hormone (LH — Reference Range Female, Follicular phase: LH — 2–10 IU/L) were measured in a serum of highly sensitive immunoradiometric assays (IRMA) using two monoclonal antibodies with kits from Immunotech, France.
Estradiol (E2 — Reference Range Female, Follicular phase: 9–550 pmol/L) is measured in a serum with Radioimmunoassay (RIA) with kits from Immunotech, France.
Anti-Müllerian hormone (AMH) was measured in a serum via manual Generation II (Gen II) ELISA method (Beckman Coulter, USA). The analysis is deemed the most sensitive today and uses two monoclonal antibodies from Diagnostic Systems Laboratories, USA. Analytical sensitivity detection limit of 0.08 ng/mL; intra-assay (3.4–5.4%) precision; inter-assay (4–5.6%) precision. Reference range method: girls–1–8.9 ng/mL.
N-MID Osteocalcin,
| Age | N – MID Osteocalcin | |
| 10–13 years old | 0.519–2.415 ng/mL | 49–167 ng/mL |
| 14–17 years old | 0.242–1.291 ng/mL | 14–85 ng/mL |
| 8–32 ng/mL |
| Serum levels of 25OHD | Classification of 25OHD status |
| Severe Deficiency | |
| 10–20 ng/mL | Deficiency |
| 20–30 ng/mL | Insufficiency |
| Sufficiency (Normal serum levels) |
Each girl was examined as per an individual program, in which a particular date was calculated for each exam on a case by case basis.
After a detailed current personal and family medical history was taken and recorded in a form (for each individual case), a final assessment was determined to evaluate the degree of development of secondary sexual characteristics according to the approved in literature and in practice in Bulgaria — Tanner scale — thelarche, pubarche, adrenarche. The presence or lack of hirsutism-moustache, under the chin, lower abdomen, sternum, and pubis — was evaluated.
Serum levels of LH, FSH, estradiol, AMH were measured during the early follicular phase of the menstrual cycle where the timing of the evaluation of the indicators was in accordance with the menstrual intervals or after an induced uterine bleeding by means of progestins (one tablet twice a day for 5 days-Lynestrenol per os). Samples were collected in the morning between 8 AM and 10 AM on an empty stomach after 30 minutes of rest before the venipuncture.
Transabdominal or transvaginal ultrasound of lesser pelvis — to determine the presence of follicles, the thickness of the uterine lining, uterine sizes. Exam was performed with MEDISON Accuvix V20 (Germany).
All data was entered and processed via statistical package IBM SPSS Statistics
25.0 (IBM Corp., Armonk, NY, USA).
The tested clinical population involves 78 girls at an average age 15.93
It can be seen on Table 3 that the two tested groups were statistically made equal in terms of the known confounding factor age which ensured correctness of the subsequent comparisons.
| Groups | Age | ||
| N | SD | ||
| Control | 42 | 15.69 |
1.62 |
| With opsomenorrhea (PCOS) | 36 | 16.11 |
1.43 |
| Note: The similar letters in vertical show lack of a significant difference and
the different ones — existence of such (p | |||
The average age for menarche (first menstruation in life) of the whole sample
was 12.38
| Group | Menarche | ||
| N | SD | ||
| Control | 42 | 12.19 |
0.51 |
| With opsomenorrhea (PCOS) | 36 | 12.53 |
0.51 |
| Whole sample | 78 | 12.38 | 0.52 |
| Note: The similar letters in vertical show lack of a significant difference and
the different ones — existence of such (p | |||
Early menarche is associated with early establishment of ovulatory cycles. When the first menstruation occurs earlier than 12 years, 50% of menstrual cycles are ovulatory in the first year after menarche. With a late menarche, it can take eight to twelve years for all menstrual cycles to become ovulatory. Later onset of the first menstruation and menstrual disorders indicate impaired function of the HHO-axis and can be an important risk factor for insufficient and inadequate accumulation of bone mass.
It is discutable whether adipose tissue contributes to the accumulation of bone mass or by various mechanisms to lead to bone loss.
A significant difference has been found in terms of BMI, and 38.9% of the
patients with PCOS were overweight, but in the control group this percentage was
9.5% (p
| Statistics | Groups | Total | ||
| Control | PCOS | p | ||
| N | 1 | 1 | ||
| % | 2.40% | 2.80% | ||
| 18.5–25.0 | N | 36 | 18 | |
| % | 85.70% | 50% | ||
| N | 4 | 14 | ||
| % | 9.50% | 38.90% | ||
| Total | N | 42 | 36 | |
| % | 100% | 100% | ||
In the group of patients with PCOS, there were statistically
significant higher serum levels of gonadotrophic hormones (LH — p
| Indicators | Control groups | PCOS | p | ||||
| N | SD | N | SD | ||||
| Osteocalcin | 42 | 39.36 | 20.68 | 36 | 23.84 | 8.83 | |
| 42 | 1.03 | 0.4 | 36 | 0.94 | 0.28 | 0.44 | |
| LH | 42 | 4.92 | 1.83 | 36 | 8.96 | 2.7 | |
| FSH | 42 | 5.23 | 1.91 | 36 | 6.71 | 2.39 | 0.017 |
| Estradiol | 41 | 444.46 | 207.31 | 36 | 356.94 | 157.91 | 0.043 |
| AMH | 42 | 5.68 | 3.25 | 36 | 10.11 | 2.67 | |
In spite of the hormonal characteristics of normogonadotropic normogonadism in those patients, the significantly lower values of osteocalcin showed a suppressed bone metabolism and in particular–bone formation compared to the healthy control groups that may be interpreted as existence of enhanced risk of insufficient bone mass accumulation and risk of early onset of osteoporosis later in life.
The high diagnostic reliability of AMH as a surrogate marker complementing the Rotterdam criteria in the diagnostic process of patients with PCOS has been proven in numerous studies.
ROC curve analysis was applied to determine the cut-off values of АМН and asses the option to use it as a surrogate marker in the diagnostic process of patients with PCOS, supplementing the accepted Rotterdam criteria of ESHRE/ASRM. The criteria for optimization in the choice of cut-off value were high sensitivity and precision. According to the calculated values of validation criteria (Table 7).
| Reason | Cut-off value | Sensitivity | Specificity | Positive predictive value | Negative predictive value | % true answers |
| PCOS | 94 | 69 | 72 | 94 | 81 |
In the patients with PCOS, sensitivity — 94% and specificity — 69% were
ascertained, with cut–off value
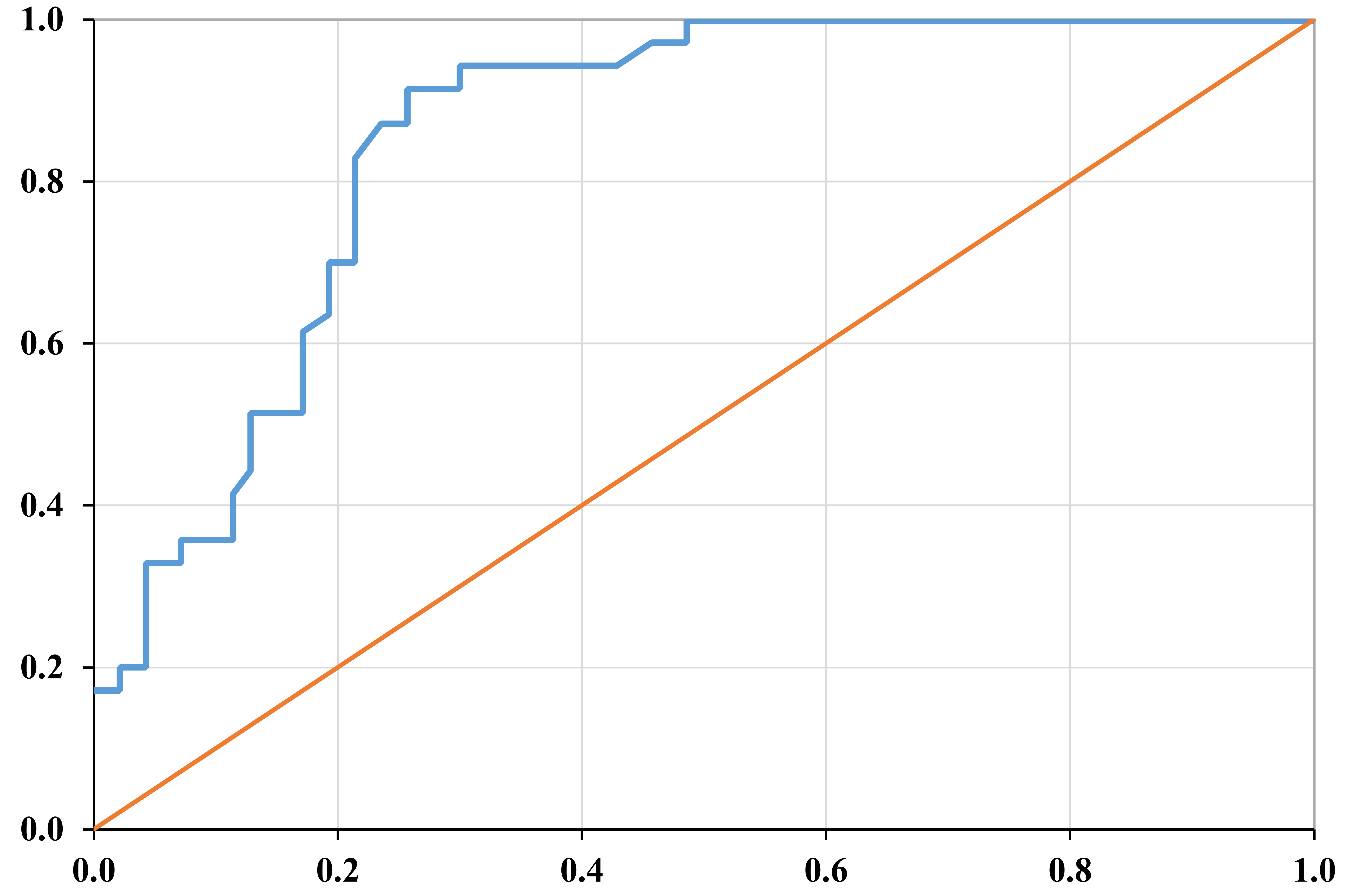 Fig. 1.
Fig. 1.ROC curve of AMH for determining its cut-off value when separating those
having PCOS from the ones of the control group (area below the curve 0.854,
p
Vitamin D plays an extremely important role in calcium-phosphorus (Ca
The performed comparative analysis of the percentage of shortage of Vitamin D (values of Vitamin D below 30 ng/mL) in both groups and in total in the studied population showed that (Table 8).
| Group | Patients with shortage of Vitamin D | ||
| N | % | Sp | |
| Healthy control groups | 25 | 59.5 |
7.6 |
| Girls with (PCOS) | 16 | 44.4 |
8.3 |
| Note: The similar letters in vertical show lack of a significant difference and
the different ones — existence of such (p | |||
No statistically significant difference between the studies group was found in terms of insufficiency of Vitamin D.
In patients with PCOS, normogonadotropic normogonadism is observed, but nevertheless, they have disorders in the menstrual cycle characterized by prolonged intermenstrual intervals. The diagnosis PCOS was determined in observance of the requirements of ESHRE/ASRM — the patients to comply with at least two of the three Rotterdam criteria [2, 3].
The average values of the gonadotropic hormones in those patients were higher
compared to the control group but were within normal reference ranges (LH —
8.96
Franks and Bednarska report in their studies that the patients with PCOS are characterized by increased serum levels of androgens, LH and AMH, but by normal or low serum levels of FSH [2, 4].
Tokmak et al. [5] publish in their study involving 90 girls, of which
43 with PCOS, and the remaining in healthy control groups, higher levels of AMH
(10.1
A prospective study performed by Park et al. [6] reports about
significantly higher levels of AMH in patients with PCOS in the period of puberty
— adolescence compared to healthy control groups. The study involves 220
patients with oligomenorrhea/opsomenorrhea, with PCOS and healthy control groups,
and the patients with oligomenorrhea were without clinical or paraclinical
evidence of androgenic excess. They find in their study increased levels of AMH
in the patients with oligomenorrhea and in those with PCOS, having androgen
excess (5.33
The serum levels of gonadotropic hormones and estradiol are comparable with the
results found by us as follows LH — 9.3
In the group of patients with PCOS, we reported statistically significant lower
values of osteocalcin (p
In their study covering 298 patients with PCOS and 194 healthy control groups,
Lingaiah et al. [25, 26] report about statistically significant lower
levels of osteocalcin in those patients but do not find any significant
difference between the levels of
On the other hand, studies conducted in adolescent patients with evidence of PCOS report about a possible protective effect of hyperinsulinemia and increased androgenic level on bones and about lack of a significant difference between patients with PCOS and healthy control groups regarding bone metabolism markers and bone mineral density [27, 28].
The high reliability of AMH as a surrogate marker supplementing Rotterdam criteria in the diagnostic process of patients with PCOS has been proven in multiple studies.
A high specificity and sensitivity (98% and 93%) are reported by Saikumar et al. [7] in their study at cut-off value of AMH 3.34 ng/mL. Woo et al. [8] report about sensitivity (75.9%) and specificity (86.8%) with a higher cut-off value of AMH 7.82 ng/mL, Lin et al. [9] — 92% — specificity and 67% — sensitivity at cut-off value of 7.3 ng/mL.
Dewailly et al. [10] report higher specificity and sensitivity (92% and 97%) with a cut-off value of 4.9 ng/mL. Homburg et al. [11] report a higher specificity (98.2%) but lower sensitivity (60%) of AMH with cut-off value of 6.7 ng/mL.
In the study performed by us, we found high sensitivity — 94% and specificity 69% with cut-off value of AMH of 5.95 ng/mL, a positive predictive value of 72% and a negative predictive value — 94%. These differences in the cut-off value maybe result from the use of various kits for AMH. Cengiz et al. [12] reach the conclusion that where AMH is used separately and not in integrity with Rotterdam criteria, it is not a reliable indicator for determining or rejecting the diagnosis of PCOS.
The increase of BMI is related to an increase of bone mineral density. A lower risk of osteoporosis fractures is reported in overweight women compared to those of normal and especially sub-normal values of BMI [13, 14].
Ravn et al. [15] compare women with obesity with women of normal body
weight at the same age and reach a conclusion that in the first group, there is a
significantly lower risk to develop osteoporosis. On the other hand, obesity may
result in bone loss by stimulating the formation and release of pro-inflammatory
cytokines-IL-6 and tumour necrosis factor-
There are a number of studies in which, no significant difference between serum
levels of Vitamin D is found in patients with PCOS and control groups [38, 39, 40]
but in other studies, statistically significant higher levels with PCOS
Lagowska studied serum levels of Vitamin D in 77 patients with opso-(PCOS) and amenorrhea (primary and secondary) and a control group of women with a normal menstrual cycle and found significantly lower values in the patients with hypomenstrual disorders compared to the control groups. She relates the observed deficit of Vitamin D in patients with PCOS with increased levels of PTH, anovulation, hyperandrogenism and infertility [43].
No statistically significant difference was found between the patients with PCOS and the healthy control groups in terms of insufficiency of Vitamin D in the study conducted by us.
Despite the hormonal characteristic of normogonadotropic normogonadism in patients with PCOS in adolescence, the significantly lower values of osteocalcin showed a suppressed bone metabolism and in particular bone formation, compared to the healthy control groups which may be interpreted as existence of an enhanced risk of insufficient bone mass accumulation and risk of early onset of osteoporosis later in life.
DH—extraction and drafting of the manuscript, analysis of data, manuscript revision; DH and GK—design and revision, statistical analysis. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Ethical approval was granted by the Ethics Committee of Scientific Research at the Medical University-Sofia (ECSRMUS) — protocol NO. 2237, May 19th, 2015. Parent (legal guardian) has signed written informed consent form for participation in the trial.
Special thanks to Milko Sirakov and Suzana Nashar for their intense and beneficial collaboration.
This research received no external funding.
The authors declare no conflict of interest.
