- Academic Editor
Background: A preclinical animal model is an imperative tool for uncovering and understanding the tumourigenic hallmarks of human ovarian cancer; the disease is often lethal because it is commonly diagnosed in the advanced stage, where widespread cancer nodules mainly reside within peritoneal regions. Mouse models as a xenograft tumour host or genetic manipulation ovarian cancer-derived mice are widely used for studying specific hypothesis rationale in ovarian cancer. However, limited information associated with disease progression is obtained from such studies; whether it is the best model to study advanced ovarian cancer phenotype or suitable preclinical biomarkers for detecting and monitoring ovarian cancer progression is under study. This study used an ID-8 syngeneic mouse ovarian cancer model with immunocompetence. We monitored cancer growth and development using combination modalities of cancer-specific cancer antigen-125 (CA-125), interleukin-6 (IL-6) and vascular endothelial growth factor (VEGF) blood markers, which are well-known for their association with tumour progression in humans. Methods: Ten C57/BL6 female mice were intraperitoneally implanted with ID-8 Trp53 wild-type and monitored the progression of the tumour, until mice developed clinical ascites. Blood was taken at the time of intraperitoneal (IP) implantation (Day 0) and then collected weekly, and levels of biomarkers were analysed with enzyme-linked immunosorbent assay (ELISA). In addition, tumour tissue was collected and proceeded with histological staining. Results: We found that blood biomarkers CA-125, IL-6 and VEGF were not readily correlated with tumour progression. However, these biomarkers were markedly elevated in ascitic fluid at the advanced stage of the disease. Conclusions: We conclude that blood biomarkers in a syngeneic mouse model are, to some extent, not readily found in the blood as opposed to human ovarian cancer. Model anatomical and physiological differences between rodents and humans might explain this discrepancy.
Murine models have provided the means to facilitate understanding tumorigenesis’s hallmarks and test novel anticancer agents and diagnostic modalities in a preclinical setting for numerous cancers, including ovarian cancer.
In ovarian cancer research, these murine models range from carcinogen-induced tumour models, genetically induced ovarian epithelial tumour models, immunodeficiency mouse models for human ovarian cancer xenograft models, and syngeneic ovarian epithelial tumour models [1, 2, 3]. Each ovarian tumour mouse model poses its strengths and weaknesses, but it is an integrative technological tool necessary for the scientific breakthrough of ovarian cancer fields.
Increased mortality in ovarian cancer is due to late diagnosis. BRCA1/2 germline mutations are widely recognised as significant genetic risk factors for epithelial ovarian cancers, with the highest known impact. These mutations are found in approximately 6–15% of women diagnosed with epithelial ovarian cancer. Understanding a patient’s BRCA1/2 status can provide valuable information for counselling regarding expected survival. Research suggests that BRCA1/2 carriers tend to respond more favourably to platinum-based chemotherapies compared to non-carriers. Consequently, BRCA1/2 carriers may experience improved survival rates, even when the disease is typically diagnosed at a later stage and higher grade [4]. In addition, advanced ovarian cancer is challenging to treat due to its complications [5]. In the study conducted, we focus specifically on epithelial ovarian cancer, which accounts for approximately 90% of all ovarian cancers. Epithelial ovarian cancer encompasses multiple histologic types, each characterised by specific molecular changes, clinical behaviours, and treatment outcomes. The remaining 10% of ovarian cancers are classified as non-epithelial, consisting mainly of germ cell tumors, sex cord-stromal tumors, and a few exceptionally rare tumors such as small cell carcinomas [6, 7].
Epithelial high-grade serous ovarian cancer (HGSOC) is considered sensitive to initial chemotherapeutic treatment [8]. However, usually, a chemoresistant phenotype will rapidly develop [9]. Having technologies capable of predicting drug response in chemoresistant ovarian cancer is crucial. Proteomic technologies, such as mass spectrometry and protein array analysis, have made significant advancements in dissecting molecular signalling events and characterising the proteomic profile of ovarian cancer. By conducting proteomics analysis of ovarian cancer and studying their adaptive responses to therapy, valuable insights can be gained. This can lead to the identification of new therapeutic options, which have the potential to minimise the development of drug resistance and ultimately enhance patient outcomes [10]. Furthermore, many novel cancer treatments modify cancer growth. Poly (ADP-ribose) polymerase (PARP) inhibitors are a prime example of targeted therapies used in ovarian cancer treatment. Mutations in DNA damage repair (DDR) genes, particularly BRCA genes, disrupt the homologous recombination (HR) pathway and contribute to aggressive disease. PARP inhibitors leverage synthetic lethality, inducing DNA breaks that cannot be efficiently repaired in HR-deficient or PARP-inhibited cancer cells, leading to their demise. Initially designed for BRCA-mutated cancers, PARP inhibitors have extended their reach to non-BRCA mutated tumours with similar vulnerabilities [11].
The syngeneic mouse ovarian cancer model is a valuable preclinical tool that mimics the early growth and invasion observed in the peritoneal cavity, closely resembling the advanced stage of ovarian cancer. This model is crucial for studying the role of the microenvironment in the spread of ovarian cancer within the peritoneum. Additionally, the microenvironment of malignant ascites, which includes fully functional immune cells, closely resembles the conditions observed in humans, making it highly relevant for studying ovarian cancer in a translational context [12, 13]. To optimise the utilisation of the syngeneic ovarian cancer model, it is essential to have reliable methods for monitoring cancer progression in vivo. In this study, we investigate the potential of various tumour marker candidates in a previously under utilised murine ovarian cancer model. By exploring these markers, we aim to identify effective tools for tracking and assessing the development and progression of ovarian cancer in the preclinical setting.
Cancer antigen-125 (CA-125) is a repetitive motif of mucin 16 protein and is the primary blood biomarker utilised at the initial detection stage to determine the risk of ovarian cancer in patients [14, 15]. Furthermore, the CA-125, combined with other clinical modalities, including transvaginal sonography (TVS), may aid in disease diagnosis. Monitoring CA-125 in the serum sample is also clinically valuable to follow ovarian cancer response to treatment. However, CA-125 sensitivity and specificity are questionable since elevated serum CA-125 levels can be found in nongynaecological malignancies and non-malignant conditions [16, 17, 18]. Therefore, CA-125 could be combined with alternative sera biomarkers to improve its specificity and sensitivity.
Interleukin-6 (IL-6) is a pleiotropic cytokine produced by various cell types, including osteoblasts, monocytes and macrophages, and is typically kept at low levels in normal physiological conditions [19]. However, several physiological factors, including diet, exercise, stress, infection, injury and inflammation, can increase IL-6 levels [20]. In addition, malignant cells can produce IL-6 and allow its stimulation in an autocrine and paracrine manner [21]. IL-6 can be found in serum and ascitic fluids in patients with advanced ovarian cancer, playing a critical role in tumour progression and metastasis [22].
Vascular endothelial growth factor (VEGF) is another cytokine and plays a crucial role in the angiogenic process in normal and pathological conditions. Many cell types produce it. In the advanced stages of ovarian cancer, patients often develop malignant ascites with peritoneal carcinomatosis, and tumour burden and ascites are significantly associated with poor prognosis and survival [14, 23, 24, 25]. Serum and ascitic fluid from ovarian cancer patients have significantly high levels of VEGF, but its correlation with a poor prognosis is controversial and seems to be VEGF isoforms dependent [26].
This study used the combination of blood biomarkers, CA-125, IL-6 and VEGF, to predict and monitor the tumour progression of a syngeneic mouse ovarian cancer implanted with ID-8 cells. We hypothesised that combining these three biomarkers could be a reliable tool to predict the onset of tumour progression in a syngeneic mouse model.
Ten C57/BL6 female mice at ages 6–8 weeks were intraperitoneally (IP) injected
with four million ID-8 cells. Before tumour implantation (Day 0), the blood of
each mouse was taken from a saphenous leg vein with a 23 G needle (Thermo Fisher, Auckland, New Zealand). The blood was
mixed with a 1:1 ratio of ice-cold 5 mM ethylenediaminetetraacetic acid (EDTA)
solution in 1
The mouse MUC16/CA-125 ELISA kit was purchased from LifeSpan BioScience
(Seattle, WA, USA). Mouse IL-6 DuoSet and mouse VEGF DuoSet were purchased from
R&D systems (Minneapolis, MN, USA). A volume of 20 µL of serum
samples was mixed with 80 µL of a sample buffer. For ascitic fluid,
20 µL of undiluted ascitic fluid was mixed with 80 µL
1
Fixed tumour nodules were embedded in square moulds (Peel-A-Way™, Sigma-Aldrich, Auckland, New Zealand) with an optimal cutting temperature (OCT) embedding medium (Thermo Scientific, Auckland, New Zealand), and samples were kept at –80 °C overnight. Sections of seven-micrometrer thickness were cut by Cryostat (Leica CM1860, Leica Biosystems, Germany). Each cut section was transferred to SuperFrost Plus microscope slides (ThermoFisher, Auckland, New Zealand). Then, tumour sections were stained with Haematoxylin & Eosin solution (Merck, Aukland, New Zealand). Dried tissue was then mounted with a mounting solution (DPX, mounting medium, Scharlau). Tumour sections were then imaged by a Zeiss Axioimager Z1 microscope (AxioVision 4.5, Carl Zeiss Oberkochen, Germany).
The ID-8 cell line was obtained from Centre for Redox Biology and Medicine,
University of Otago, Christchurch, New Zealand. The OV-90 was obtained from
Discipline of Obstetrics and Gynaecology, Robinson Research Institute, Adelaide
Medical School, the University of Adelaide, Adelaide, Australia. ID-8 and OV-90
cell lines was tested for the mycoplasma contamination by polymerase chain
reaction (PCR) assay using generic primers GPO-3/MGSO (270 bp). The ID-8 cell was
been authenticated by cell morphology. The OV-90 cell line had been authenticated
using STR testing by CellBank (Children’s Medical Research Institute, New South
Wales, Austratia). ID-8 and OV-90 cell lines were fixed with cold acetone:
methanol (50%:50%) (Merck, Auckland, New Zealand) solution for 40 min at 4 °C. Sections of tumour
tissues and cell lines were washed with 1
Each mouse exhibits distinct signs of tumour progression at different time points. However, with the exception of mouse number 10, all other mice show advanced-stage cancer progression characterised by ascites (Table 1). Mouse number 10, on the other hand, presents small tumour nodules on the mesentery and no signs of abdominal wall growth. Consequently, we conducted a peritoneal wash on mouse number 10 to obtain any remaining peritoneal fluid for further analysis.
| Mice ID | Weight of mice before tumour implanation (g) | Weight of mice before culling (g) | Total weight increased (g) | Weight increased (%) | Volume of ascites (mL) | Time of survival (days) |
| 1 | 18.5 | 27.7 | 9.2 | 49.7 | 7.0 | 88 |
| 2 | 18.5 | 23.4 | 4.9 | 26.4 | 3.0 | 90 |
| 3 | 20.3 | 24.2 | 3.9 | 19.2 | 8.0 | 81 |
| 4 | 19.1 | 27.6 | 8.5 | 44.5 | 5.0 | 94 |
| 5 | 18.6 | 25.6 | 7.0 | 37.6 | 5.0 | 91 |
| 6 | 15.3 | 20.8 | 5.5 | 35.9 | 3.5 | 96 |
| 7 | 15.5 | 21.3 | 5.8 | 37.4 | 2.0 | 96 |
| 8 | 18.0 | 23.0 | 5.0 | 27.8 | 4.0 | 84 |
| 9 | 17.0 | 21.2 | 3.7 | 21.7 | 3.5 | 82 |
| 10 | 17.5 | 19.2 | 1.7 | 9.7 | 0 | 112 |
Mice numbered 1 to 9, which developed ascites, experienced shorter survival times and succumbed to the disease between 81 to 96 days. We also analysed the weight increase of mice before tumour implantation and after the development of ascites. While we observed varied weight gains, it is important to note that we cannot definitively attribute these changes solely to the presence of tumours, as we lack control mice without tumours for comparison.
To investigate the potential association between tumour progression and increased total body weight, we conducted weekly weight measurements of each mouse following intraperitoneal implantation of tumour cells. As depicted in Fig. 1, over the course of 60 days, the weight of all mice exhibited fluctuations and a gradual increase. However, starting from day 70, nine out of ten mice (M1–M9: 90%) displayed a consistent and gradual weight gain ranging from 19.2% to 49.7%. In contrast, mouse number 10 exhibited a weight increase of approximately 9.7%. This may suggest that the weight change observed in mouse number 10 may be attributed to a regular growth pattern rather than tumour progression. It is important to note that we did not have control mice without tumour implantation to compare with our study. Therefore, the observed weight increase could potentially be a result of the normal growth pattern in mice.
 Fig. 1.
Fig. 1.Weights of ten mice after post intraperitoneal injection of ID-8 cells. Each animal was weighed weekly until a sign of abdominal distention due to ascites was observed. The final weight of each mouse was documented before humane euthanasia. M#1–9 developed ascitic fluid. IP, intraperitoneal.
Also, mice with ascites showed white feet, suggesting low red blood cells in blood circulation (Fig. 2). Ascites was relatively rapid and occurred within 2–3 days without any sign of enlarging abdominal girth to an enlarged abdomen. Mice with abdominal distension were still mobile and maintained their appetite.
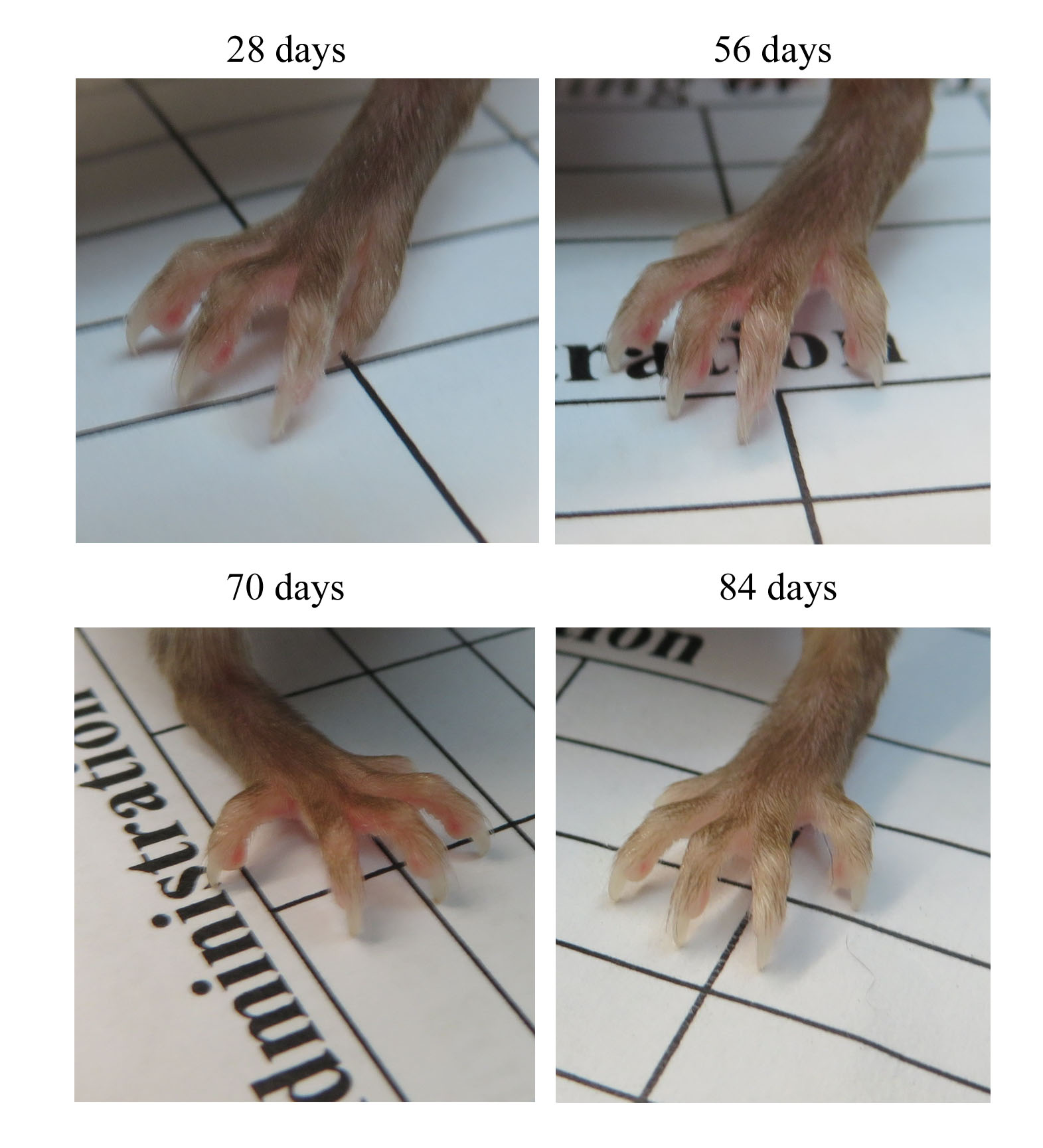 Fig. 2.
Fig. 2.Images of the animals’ feet were recorded after tumour implantation and before culling the animal. For example, the anaemic foot of a mouse on day 84 was due to ascites in the abdominal cavity. The selective images are representative of all mice that commonly have white feet associated with ascites.
As shown in Fig. 3, a mouse with ascites has an enlarged abdomen (Fig. 3A). Gross tumour examination showed extensive peritoneal growth consisting of nodules and layers (Fig. 3B). The most common metastatic sites were the surface of the abdominal cavity (Ab), mesentery (MS), omentum/pancreas (OT/PC), diaphragm, and kidneys. Rare but uncommon sites were the surface of the spleen (SPL), colon, liver and ovary. All mice (other than mouse 10) had blood-stained ascitic fluid, and the fluid volume varied among mice (Table 1 and Fig. 3C). Also, clusters of ID-8 cells were present in the ascitic fluid (Fig. 3D).
 Fig. 3.
Fig. 3.The anatomical and pathological appearance of a mouse after developing ascitic fluid. The image shows abdomen distension due to ascites (A), extensive tumour spread within a peritoneal cavity preferentially to the abdominal surface (Ab), mesentery (MS), and omentum/pancreas (OT/PC) but rarely growing on the surface of the spleen (SPL) (B), ascitic fluid contains red blood cells (C) and clusters of ID-8 cells were present in the ascitic fluid (D). Chosen images in (A–C) are representative of all mice that have abdominal distension due to ascites, tumour burdens and haemorrhagic ascites of advanced ovarian cancer.
Tumour nodules and layers were found in various places within the peritoneal cavity, and common places are the surface of the abdominal wall (Fig. 4A), mesentery (Fig. 4B), omentum/pancreas (Fig. 4C) and diaphragm (Fig. 4D).
 Fig. 4.
Fig. 4.Histology of ID-8 tumour in mice that grow on various location of the internal organs. Haematoxylin and Eosin (H&E) staining of ID-8 tumour nodules and layers on the surface of the abdominal wall (A), mesentery (B), omentum/pancreas (C), and diaphragm (D). The black arrows point at tumour nodules and layers.
As shown in Fig. 5, levels of all three sera biomarkers, CA-125 (Fig. 5A), IL-6 (Fig. 5B), and VEGF (Fig. 5C), fluctuated and did not elevate before mice developed ascites. However, these three biomarkers were elevated in ascitic fluid (Fig. 5D). The level of ascitic CA-125, IL-6, and VEGF are high relative to their levels in the blood. Mouse number 10 had small tumour nodules on the mesentery and no ascitic fluid. Therefore, we performed a peritoneal wash on mouse 10 to obtain peritoneal fluid and use it to detect IL-6.
 Fig. 5.
Fig. 5.Levels of biomarkers in blood and ascitic fluids of mice with tumour. Levels of CA-125 (A), IL-6 (B), VEGF (C) and ascitic fluid biomarkers (D) of mice implanted with ID-8 cells. Blood and ascitic fluid samples from mice were detected for biomarkers using enzyme-linked immunosorbent assay (ELISA). The levels of three markers were highly detectable in ascitic fluids (D). After culling mice, ascitic fluid from 9 mice (M#1–9) and peritoneal wash of one mouse (M#10) was subjected to IL-6 detection. Ascitic fluid from six mice (M#1–6) was employed for CA-125 detection. Ascitic fluid from three mice (M#2, 3, and 4) was used to detect vascular endothelial growth factor (VEGF). CA125, cancer antigen-125; IL-6, interleukin-6; VEGF, vascular endothelial growth factor.
The cellular location of CA-125 is a part of the mucin-16 protein, reflects its biological functions and is an important marker for ovarian cancer. Therefore, we want to determine the presence of mucin-16 in mouse ovarian cancer ID-8 cells compared to a human ovarian cancer cell line, OV-90. OV-90 (Fig. 6A) and ID-8 (Fig. 6B) cell monolayers have mucin-16 in the nucleus and plasma membrane. However, the ID-8 tumour tissues (Fig. 6C,D) showed intense staining of mucin-16 at the cell surface of the plasma membrane but less evidence of the nucleus-associated staining.
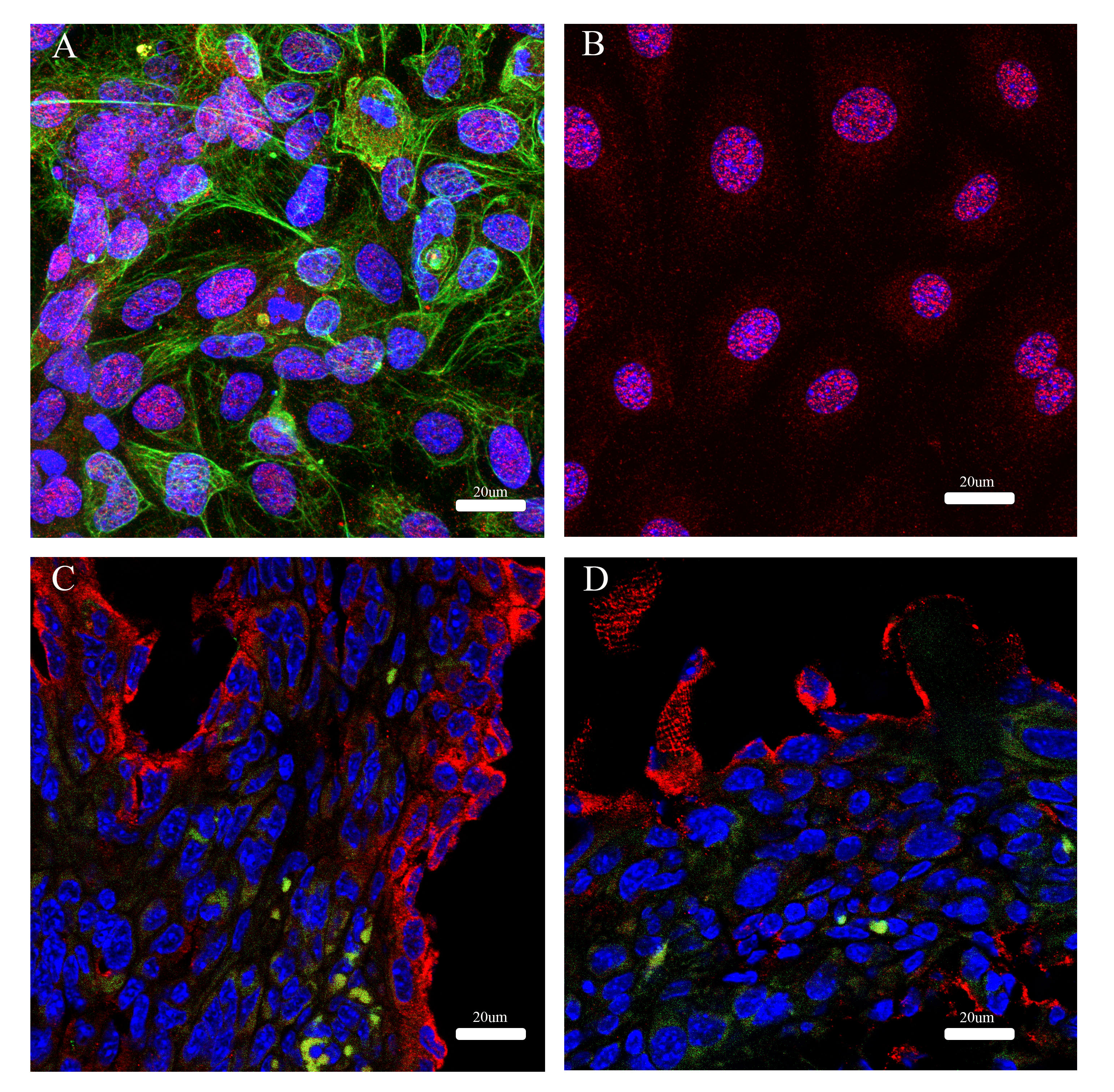 Fig. 6.
Fig. 6.Immunofluorescence of Mucin-16 and cytokeratin-18 in cell monolayers and cells in tumour tissue. Immunofluorescence of Mucin-6 (red) and cytokeratin-18 (green) in a human ovarian cancer cell line, OV-90 (A) and a mouse ovarian cancer cell line, ID-8 (B). Tumour tissue of ID-8 in a mouse model was from the omentum/pancreas (C) and the surface of the abdominal wall (D).
This study demonstrates the feasibility of using the three biomarkers, CA-125, IL-6 and VEGF, to monitor the tumour progression of a syngeneic ovarian ID-8 tumour model. Although these three blood biomarkers are not readily correlated with the ID-8 tumour progression, high levels of these biomarkers are readily detectable in ascitic fluid. The syngeneic ID-8 tumour model enables the exploration of pathways that potentially contribute to chemoresistance during the advanced stages, mirroring the presence of ascitic fluid, which is commonly observed in patients with advanced ovarian cancer. Ascitic fluid, being a crucial biological sample, plays a pivotal role in tumour progression and significantly influences the efficacy of cytotoxic and targeted agents used in ovarian cancer treatment [27]. Hence, this area of research deserves immediate attention and investment in the field of ovarian cancer.
Detection and monitoring ovarian tumours with peritoneal spread poses immense
challenges in preclinical and clinical settings. Unfortunately, routine body
examination does not readily recognise silent symptoms associated with ovarian
tumour progression. Therefore, the primary clinical practice for detecting tumour
progression is to use a blood biomarker, CA-125. The CA-125 is referred to as the
N-terminal part of the extracellular domain of Mucin 16 (MUC16) and is cleaved
and released into circulation. It was initially believed to be a specific
biomarker for ovarian cancer. However, CA-125 is found in several pathological
conditions [16, 18, 28]. Patients with ovarian cancer have significant detectable
levels of CA-125 in serum and ascitic fluid. In addition, the level of CA-125 in
ovarian cancer patients is higher in ascitic fluid in serum [29]. A high level of
CA-125 is strongly associated with advanced-stage disease, suggesting MUC16 might
play a key role in tumourigenic progression, which is facilitated by the
cytoplasmic domain of MUC16 that have phosphorylation activity. However, little
is known about how the MUC16 cytoplasmic domain phosphorylation conveys cell
signalling or participates with other cellular proteins. A recent study by Liu
et al. [17] shows that knocking down the expression of MUC16 mediated
via the Janus Kinase (JAK) pathway
significantly inhibits the growth and metastasis of colorectal cancer cells
in vitro and in vivo. Interestingly, CA-125 and MUC16 can be
found in all ovarian cancer subtypes, suggesting that the underlying tumorigenic
phenotype’s biological aspects could be molecular subtype-independent, and it
might be an excellent cellular target for treatment intervention. A previous
study showed that engrafted ovarian tumour cells from ovarian cancer patients
into NOD-SCID IL2R
IL-6 is a biomarker for ovarian cancer, and patients with high serum IL-6 correlate with a poor prognosis and more chemoresistant tumour phenotype. Interleukins (IL) exhibit both positive and negative effects on endothelial cells and angiogenesis. For instance, IL-6 and IL-8 are associated with increased angiogenesis, while IL-27 and IL-10 are linked to angiogenesis suppression. However, IL-27’s antiangiogenic properties are not direct but rather mediated through the downregulation of proangiogenic-related genes such as vascular endothelial growth factor receptor-1 (VEGFR1/FLT1), prostaglandin G/H synthase 1 (PTGS1/COX-1), and fibroblast growth factor receptor 3 (FGFR3), as well as the upregulation of antiangiogenic genes like C-X-C motif chemokine 10 (CXCL10) and tissue inhibitor of metalloproteinase 3 (TIMP3). Similarly, IL-10 negatively affects proangiogenic cells, such as activated macrophages, by inhibiting proangiogenic matrix metalloproteinase 2 (MMP2) and promoting the overexpression of tissue inhibitor of metalloproteinase 1 (TIMP1) [31]. These mechanisms contribute to the complex interplay between ILs and angiogenesis regulation. IL-6 levels are significantly high in ascitic fluid and significantly associated with shorter progression-free survival [32]. The level of IL-6 from 18 ovarian cancer patients in ascites is more prevalent than in respective peripheral sera, and the IL-6 is likely a critical factor in the function of effector T cells mediated by tumor necrosis factor receptor 2 (TNFR2) [33]. Furthermore, preclinical and clinical study showed that IL-6 staining in ovarian tumour tissue was significantly associated with poor prognosis, and treatment of ovarian cancer cells with siltuximab inhibited tumour growth in tumour xenograft and patients [34]. Unfortunately, a small single-arm phase 2 clinical trial using monoclonal antibodies to block IL-6 molecules had modest clinical benefits [34].
VEGF is another serum biomarker associated with a poor prognosis in advanced ovarian cancer patients. The VEGF level is higher in serum in advanced-stage ovarian cancer patients compared to patients with benign conditions [35]. Furthermore, a specific isoform of VEGF is significantly associated with tumour lymphatic metastatic phenotype [36]. Circulating VEGF is produced from various cell types, including tumour cells and platelets. VEGF binds to its receptors, namely vascular endothelial growth factor receptor (VEGFR1, VEGFR2, VEGFR3) on blood and lymphatic vessel endothelial cells. The binding of VEGF to its receptors triggers a cascade of cell signalling pathways that facilitate a new formation of blood vessels and increase the peritoneal lymphatic and vasculature permeability [37, 38]. As a result, intraperitoneal bevacizumab dramatically improves in controlling ascites and the quality of life in patients with advanced ovarian cancer [39]. However, there are contradictory findings regarding the predictive and prognostic value of VEGF levels in ascites that did not predict progression-free survival (PFS) and overall survival (OS) in ovarian cancer [40, 41].
A previous study by Cho et al. [42] showed characteristics and tumour progression in a syngeneic orthotopic mouse ovarian cancer model of ID-8 cells using non-invasive ultrasound, clinical parameters and tumour biopsies. However, the study did not analyse blood and ascitic fluid biomarkers that are clinically relevant. Some of our results are similar to this study to some extent. However, we use clinically relevant biomarkers to detect tumour progression and clinical observations. Our study shows that CA-125, IL-6 and VEGF are not elevated in the blood associated with tumour progression. This finding contrasts these three biomarkers in advanced ovarian cancer in humans. In our study, CA-125 is the highest maker in ascites compared to IL-6 and VEGF. Therefore, we would expect to detect CA-125 in blood, but it was not the case. One speculation could be that CA-125 protein fragments in a mouse could be slightly distinct from their human counterpart. One study showed that human MUC16 is present on the cell surface, and its CA-125 soluble fraction is cleaved and shed from the cell membrane [43]. However, murine MUC16 is present intracellularly, and the fraction CA-125 is intracellularly cleaved, shed and secreted out [43]. Our data in Fig. 6 show cell monolayers of a human ovarian cancer cell line, OV-90 and murine ID-8 cells; the mucin-16 immunological staining is noticeable in the cytoplasm and the nucleus regions. However, MUC16 in ID-8 tumour tissue is starkly different from the in vitro cell monolayer, likely having the plasma membrane-cytoplasm associated with MUC16. However, this notion does not explain a lack of elevated IL-6 and VEGF levels in the blood. It might be hypothesised that the physiological contribution of anatomical constraint in a mouse peritoneum might be responsible for this. This hypothesis requires further investigation.
In humans, the peritoneum is well supplied with blood vessels and lymphatics, which generate a dense capillary network. About 70% of the plasma in the arterial capillary end will extravasate by the net balance between the hydrostatic and oncotic pressure to generate the interstitial and lymphatic fluid [44]. As a result, the healthy peritoneal cavity should contain a small volume (20 mL) of serous exudate with various free-floating cells, including macrophages, mesothelial cells, and lymphocytes [45]. A change in the microenvironment within the peritoneum caused by various conditions can tip the balance leading to a pathological state [46]. Ascites occur when tumour cells grow on the surface of the peritoneal cavity, cytokines such as VEGF and IL-8 are released from the tumour, and normal cells interrupt the normal blood and lymphatic vessels, allowing the vessels to become permeable rather than absorptive function [45, 47, 48]. The presence of protein albumin in ascites is a prominent marker of vascular leakage, a clinical sign of malignant ascites [49, 50]. It is uncommon to find red blood cells in ascites in patients with ovarian cancer, but the high amount of red blood cells in mouse ovarian tumour models is prevalent. Furthermore, mice with ascites also associate with white feet incidence, suggesting abnormally low red blood cells in the circulation.
Interestingly, in a mouse model, both syngeneic and immunocompromised mice with intraperitoneal tumour implantations produced bloody ascites [13, 51, 52].
Alternative imaging techniques use the ID-8 syngeneic tumour model to uncover tumour progression and the immune system’s role. However, these imaging modalities pose some hurdles. For instance, the study describes that ascitic fluid can compromise optical imaging for detecting red fluorescent protein-tagged ID-8 cells for metastatic process using the mice harbouring ID-8 tumours [53]. Therefore, in order to optimise the use of this murine cancer model, further research should focus on determining appropriate methods of tumour monitoring.
We acknowledge the limitations of our study. Firstly, we did not include a control group of healthy mice without tumour implantation to use as a comparison for evaluating the association between weight gain and tumour progression. It is possible that both healthy mice and tumour-bearing mice exhibit similar weight gain as an endpoint, despite potential differences in their underlying mechanisms. It is worth noting that healthy mice and tumour-bearing mice may have different appetites for food, with healthy mice following a regular growth pattern, while mice with tumours may experience irregular appetite due to reduced food intake. Consequently, the mechanical pathway of weight gain may differ between the two groups, but ultimately lead to a similar endpoint. Secondly, we acknowledge that the amount of VEGF in serum was obtained from only three mice, which may not be sufficient to draw definitive conclusions. This limitation arises from the challenge of obtaining an adequate amount of blood from the mice for analysis. The limited sample size may introduce potential variability and reduce the accuracy of our findings regarding serum VEGF levels.
The ID-8 syngeneic mouse model has been widely used in ovarian cancer research, but our study is unique in investigating the association of biomarkers with tumour progression in this model. Our findings suggest that the three biomarkers evaluated in our study are unreliable for the early detection of tumour progression in blood. However, they may be valuable in investigating ascitic fluid as a part of the unique microenvironment of the advanced ovarian cancer model. In order to obtain a clearer understanding of the syngeneic mouse model of ID-8 tumours and its ability to accurately mimic the progression of human ovarian cancer, future studies should consider incorporating control mice without tumours. By including control mice alongside the experimental group, the outcomes of the study can be more effectively evaluated. Additionally, providing information on the number of mice in both the control and experimental arms would contribute to the robustness of the findings. These considerations will enhance our ability to assess the utility of the syngeneic mouse model in predicting the in vivo progression of ovarian cancer, thus closely emulating the human disease.
CA-125, Cancer antigen 125; IL-6, Interleukine-6; VEGF, Vascular Endothelial Growth Factor; ELISA, Enzyme-Linked Immunosorbent Assay; HGSOC, High-grade serous ovarian cancer; TVS, Transvaginal sonography; IP, intraperitoneal injection; PBS, Phosphate buffer saline; rpm, rate per minute; OCT, Optimal cutting temperature.
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
KC designed, executed, and analysed the research study. AD performed an animal study. PS designed and wrote the manuscript. KC, AD and PS contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was approved by the Animal Ethics Committee of the University of Otago-Christchurch (approval number: C6/16), and this work and it’s approval was in accordance with the New Zealand Animal Welfare Act 1999, and Amendment No. 2, 2015.
We like to thank Erin Lally for maintaining animal care.
This research received no external funding.
The authors declare no conflict of interest. Kenny Chitcholtan is serving as one of the Editorial Board members and Guest editors of this journal. We declare that Kenny Chitcholtan had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Michael H. Dahan.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
