- Academic Editor
†These authors contributed equally.
Objective: To identify the impact caused by the use of assisted reproduction treatments such as in vitro fertilization (IVF), artificial insemination and ovulation-inducing drugs on the incidence of ectopic pregnancy, defined as the implantation of a fertilized ovum in a place other than the endometrium in the uterine cavity, the fallopian tube being the most frequent location. Ectopic pregnancy is considered a serious health problem for the female population of reproductive age, since it hinders fertility and increases the risk of maternal death, the main complication being hypovolemic shock associated with rupture of the ectopic gestation. Mechanism: The databases Scopus, PubMed, Web of Science and Google Scholar were searched for published studies on the incidence of ectopic pregnancy related to the use of fertility treatments. The following keywords were used: “Reproductive techniques”, “Ectopic pregnancy”, “Risk factors”, and “Infertility”. Findings in Brief: The incidence of ectopic gestations increased from 2.1% to 9.4% of pregnancies following assisted reproductive techniques. Drugs related to ovarian stimulation have been reported to increase the risk of ectopic pregnancy by up to 7.9% for clomiphene citrate and gonadotropins and 6% for aromatase inhibitors (letrozole). The use of in vitro fertilization brought an increased risk of up to 9.3 times, to a rate of 9.4% in the case of the use of intracytoplasmic sperm injection and 8.6% for embryo transfer, compared to the rates reported in natural pregnancies (1.9%). Conclusion: Surveillance, follow-up and identification of risk factors associated with assisted reproductive technologies (ART) by medical professionals are essential to timely detect ectopic pregnancy, avoid serious complications, or otherwise identify the best ART to provide patients with the lowest risk of ectopic gestations, as ART remains a valuable option for many couples who wish to conceive.
The development of a blastocyst that implants in a location other than the endometrium or outside the uterine cavity is known as ectopic pregnancy (EP). The most common extrauterine location is the fallopian tube, which accounts for 96% to 98% of all ectopic gestations [1, 2, 3, 4].
The prevalence of ectopic pregnancy ranges between 1% and 2% and has increased thanks to the use of assisted reproduction techniques (ART). In Mexico, its incidence ranges from 1.6 to 2 ectopic pregnancies per 100 births. The most frequent location is the fallopian tube, with 95% of cases located mainly in the ampullary region and the isthmus [4]. Among the nontubal forms of ectopic gestation are cornual pregnancy, with 3% of the cases, followed by abdominal pregnancy, with 1.3%, pregnancy of ovarian location, with 0.5%, intraligamentary and cervical pregnancy, both with 0.1%, and pregnancy in the rudimentary uterine horn (Fig. 1, Ref. [1, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16]).
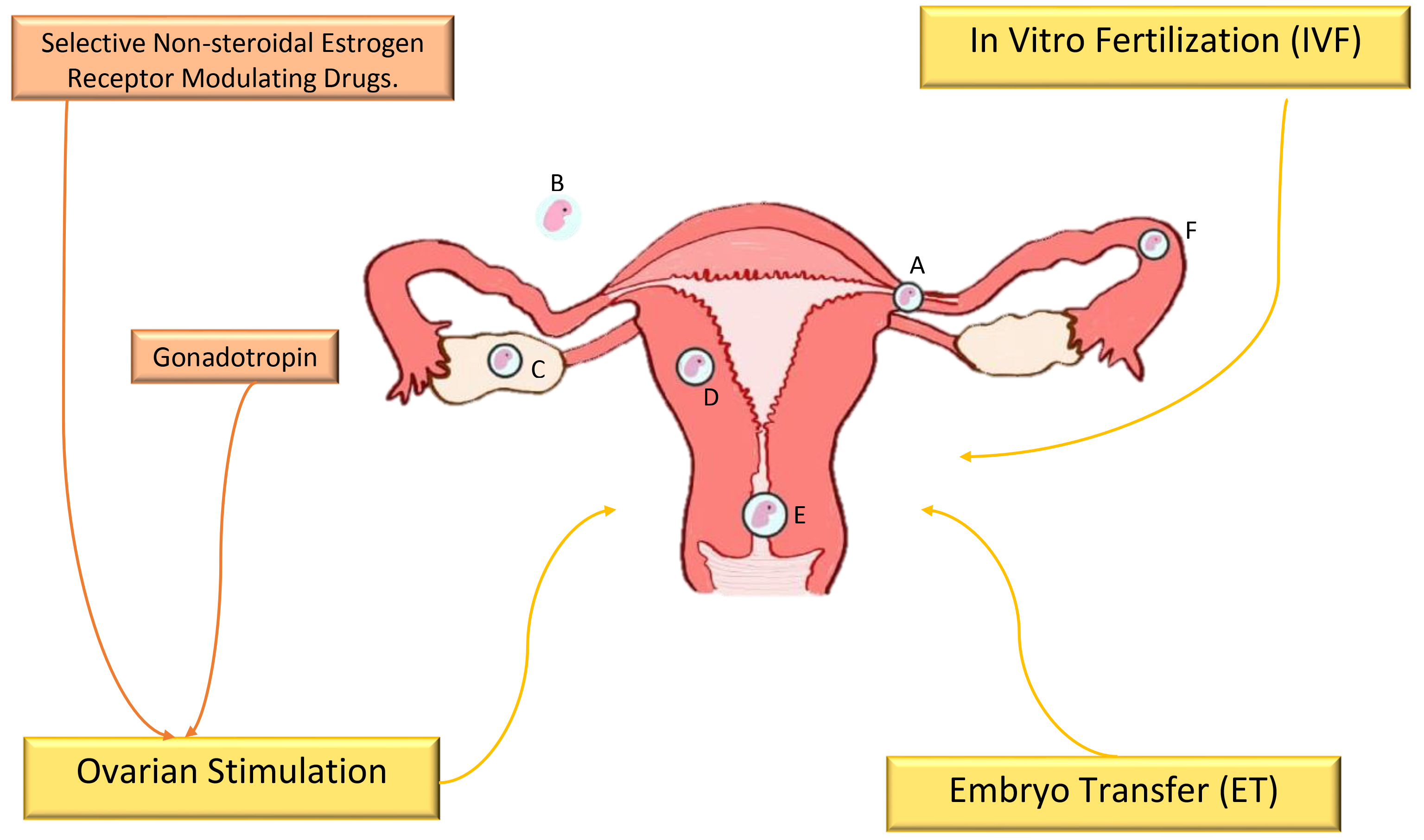 Fig. 1.
Fig. 1.The incidence of ectopic pregnancy is associated with fertility
treatments [1, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16]. Nontubal forms of ectopic gestation: A, cornual
pregnancy (3%); B, abdominal pregnancy (1.3%); C, ovarian localization
pregnancy (0.5%); D, intramural pregnancy (
A potentially fatal obstetrical emergency, associated with hypovolemic shock resulting from ruptured ectopic pregnancy in 15–20% of cases, ectopic pregnancy is considered the leading cause of maternal morbidity and mortality worldwide during the first trimester of pregnancy, with a 2.7% mortality rate, representing 5% and 10% of all maternal deaths in high- and low-resource countries, respectively, with a pregnancy-related mortality of 31.9 deaths per 100,000 pregnancies [1, 3, 6, 7, 11, 17, 18, 19, 20, 21, 22, 23, 24].
The aim of this manuscript is to provide information on the risk factors that have been associated with assisted reproduction techniques and the incidence of ectopic pregnancy. We highlight the importance of knowing these factors and, if possible, modifying them or, failing that, opting for different alternatives to lower the incidence of this pathology.
A review of the literature registered in Scopus, PubMed, Web of Science, and Google Scholar databases was conducted to obtain published manuscripts on ectopic pregnancy and assisted reproductive treatments through November 2022. No time limits for publication were set, and all types of articles in the English and Spanish languages were included.
The search term Assisted Reproduction Techniques AND Ectopic pregnancy AND Risk Factors AND Infertility was used.
The Mix Methods Appraisal Tool (MMAT) was used to quality determination. The studies were analyzed for two authors in the following domains: clear research questions, adequate data collection, appropriate quantitative approach, appropriate methods to obtain the data, validation and interpretation of recorded data, appropriate statistical analysis and interpretation. A third author resolve any conflicts.
An extrauterine gestation located in the fallopian tube occurs mainly due to factors that cause damage to the mucosa, making impossible or delaying the passage of the fertilized oocyte to the uterus, or to factors related to premature implantation [15, 25]. Chronic salpingitis has been related to ectopic gestation located in the fallopian tube, being identified in up to 90% of pathological samples, which contain plasma cells and lymphocytes responsible for inflammation of the wall [25].
Pregnancies located in the ampullary region are mainly located in the lumen of the salpingeum (in up to 85%), without causing damage to the musculature, contrary to those located in the isthmic region (intraluminal and extraluminal), which are associated with a greater area of rupture of the tubal wall, suggesting an earlier tubal implantation [26].
The transport and communication between the embryo and the ectopic site of implantation is regulated by elevated levels of estrogens and progestogens. Biologically active molecules act in an autocrine and paracrine manner, coupled with signals that activate the blastocyst and sensitize epithelial cells to the implantation of the trophoblast in extrauterine sites (e.g., cervical tissue and salpinges), generating decidualization. In the case of retention or delayed embryo transport in the salpingeum, this epithelium may provide an endometrial-like window of implantation, allowing trophoblast attachment [15].
A low percentage of ectopic pregnancies are located in the proximal portion of the salpingeum, immersed in the myometrium, called interstitial or cornual pregnancy, where the embryo directly contacts the ascending branch of the uterine artery. This branch is an accommodating site for the embryo thanks to its greater thickness of the uterine wall, allowing a more advanced gestational age, which increases the risk of hemorrhage and uterine rupture that occurs in 20% of cases at a gestational age greater than 12 weeks. To make an ultrasound diagnosis, the findings should include an empty uterine cavity, a location of the gestational sac 1 cm eccentric or lateral to the lateral border, decreased thickness (less than 5 mm), asymmetry or incomplete presentation of the myometrial bed surrounding the chorionic sac [27, 28].
A pregnancy located in the abdominal cavity can implant in the omentum, the broad ligament, large-caliber blood vessels and abdominal organs such as the intestine, where it can invade their musculature and cause microperforations, a phenomenon that is aggravated when the patient has a history of adhesions from previous surgeries, previous peritoneal implantations or pathologies that favor the development of an inflammatory environment around these organs [29]. It is called primary abdominal pregnancy when fertilization occurs directly in the abdominal cavity and secondary when it is the result of the expulsion of the fertilized ovum from the salpingeum. At a gestational age greater than 20 weeks, it is considered an advanced abdominal pregnancy, which is correlated with a large number of maternal-fetal complications, such as coagulopathy, embolism, fistulization, maternal hemorrhage and fetal malformations in up to 40% of cases [30].
A minority of ectopic pregnancies may be lodged within the cervical canal (below the internal cervical os), accompanied by an increase in size of the cervix and uterine cavity, the presence of amorphous and diffuse echoes inside the uterus, an absence of intrauterine pregnancy, the presence of the embryo sac and a placenta located superior to the external cervical os. It is important to differentiate it from a spontaneous abortion by characterizing the vascular fixation and cardiac activity. Risk factors from assisted reproductive techniques such as in vitro fertilization and from previous exposure to diethylstilbesterol have been associated with cervical canal pregnancies [31, 32].
Case fatality rates have decreased due to improved diagnosis and treatment of ectopic pregnancy. The clinician should have a high diagnostic suspicion in women of reproductive age with abnormal vaginal bleeding, acute pelvic pain and the presence of palpable adnexal mass, with 5 to 8 weeks of secondary amenorrhea, but this is not an accurate cutoff figure due to the dependence of the extrauterine location of the gestation, since it can be later if it is located in a place other than the salpingeum [33, 34].
Transvaginal ultrasound is considered the method of choice for the diagnosis of ectopic gestation due to its high tolerance, availability and sensitivity. It most often shows an adnexal mass with features suggestive of EP, such as the presence of a tubal ring or yolk sac, with a record of cardiac activity in the mass in less than 10%, although in a limited number of patients it can be diagnosed only by the presence of free fluid in the peritoneal cavity, caused by the rupture of the ectopic embryo (15%). However, implantation in the endometrial cavity does not exclude the diagnosis, especially in patients who resort to assisted reproductive techniques, in whom heterotopic pregnancies may occur in 1 in 100 pregnancies [4, 28, 33, 35, 36, 37].
Measurement of beta-human chorionic gonadotropin hormone (
Ectopic gestation can be handled by expectant management, by drugs such as methotrexate, or by surgery, depending on the circumstances of each patient [33].
Expectant management is indicated in patients who are pain-free, clinically
stable, with a gestational sac
The folic acid antagonist methotrexate modifies cell division and DNA synthesis.
It has success rates of curing ectopic pregnancy of up to 95%, and is indicated
for patients who can maintain medical follow-up; who have no hemodynamic
compromise, pain or data suggesting rupture of the ectopic gestation; who have an
adnexal tumor smaller than 35 mm; who have no report of fetal heartbeat; who have
a serum
Another alternative is ultrasound-guided local injection of 20% potassium chloride (KCl) applied intrasacularly, which inactivates the embryo. This can be done, for example, in cases of heterotopic pregnancy, as long as it meets the requirements for conservative management, using the same route of administration as methotrexate, but not in cases of heterotopic pregnancy. It is administered at a dose of 1 mg/kg with a repetition schedule at 7, 14 and 21 days [41, 42, 43, 44, 45, 46].
Surgical treatment should be done in patients who do not meet watchful waiting criteria, in whom hCG greater than 5000 mIU/mL, data suggestive of hemoperitoneum or hemodynamic instability, heterotopic pregnancy, impossibility of follow-up, contraindication or treatment failure are found [47].
In patients with risk factors that impair fertility, such as previous ectopic
pregnancy, previous pelvic inflammatory disease or a history of abdominal
surgery, it is recommended to choose salpingostomy, which better preserves
fertility (75%) than salpingectomy, which reduces the likelihood of clinical
pregnancy by up to 65%. These two options result in an ectopic pregnancy
recurrence rate of 8 and 5%, respectively, and salpingostomy brings the risk of
incomplete trophoblast removal, for which the prophylactic administration of
methotrexate and weekly monitoring of
Several risk factors have been associated with gestation outside the uterine cavity, especially the use of assisted reproduction techniques, fertility treatments, and certain contraceptive methods such as intrauterine devices (IUDs), as well as pelvic inflammatory disease (PID). PID is one of the most important causes of alterations in the tubal anatomy, mainly due to infectious, surgical, congenital anomalies or tumors, hormonal or immunological factors, causing a functional deterioration with affectation of the ciliary activity. In addition to maternal age over 30 years, smoking, early onset of sexual activity, number of sexual partners, douching, history of previous miscarriage or ectopic pregnancy, endometriosis, history of previous pelvic surgery and female sterilization are risk factors for ectopic pregnancy. These risk factors are not necessarily independent of each other [2, 3, 5, 11, 17, 18, 22, 31, 49, 50, 51, 52, 53, 54, 55].
The risk of ectopic gestation is three to eight times higher in patients with a history of previous ectopic pregnancy due to the underlying cause and the treatment of choice for the initial ectopic pregnancy. Opting for tubal-sparing treatment strategies has a favorable impact on fertility but contributes to a higher recurrence of ectopic pregnancy [2, 5, 14, 16, 21, 49].
In turn, preceding infertility and previous adnexal surgery have been identified as important risk factors for recurrence of ectopic pregnancy, probably explained by the underlying tubal damage and that caused by infertility treatment [2, 5, 17, 21, 23, 47, 53]. In contrast, multiparity reduces the likelihood of recurrence, along with condom use, because it limits the possibility of sexually transmitted infections (STIs) and their risk of producing PID with their respective complications [21]. Gestations following early EP have a significantly increased risk of preeclampsia, preterm delivery, and emergency cesarean delivery compared to previous pregnancies that ended with live birth; however, this risk is not significantly higher than those of other types of early first-gestational losses [2].
Infectious processes are one of the main causes of tubal pathology, bringing with it an increased risk of ectopic pregnancy. Chlamydia trachomatis (CT) causes EP in up to 60% of reproductive-age women whom it infects due to the damage it causes in the tubal anatomy by provoking a local inflammatory response, altering the ciliary activity of the tubal epithelium and the contraction of the tubal smooth muscle that can stop the transport of the embryo through the oviduct, producing tubal obstruction, pelvic adhesions and pelvic inflammatory processes [2, 3, 11, 14, 17, 21, 56].
Infertility is a condition of the male or female reproductive system that results in the inability of the female partner to get pregnant after 12 months or more of regular unprotected intercourse and is considered a secondary condition to the inability to get pregnant after a previous conception [57].
A low percentage of infertile women (5–10%) may have underlying genetic abnormalities, such as chromosomal aberrations, genetic mutations, and polymorphisms, together with factors such as ovulation disorders; anatomical alterations affecting structures such as the fallopian tubes and uterine lesions; exposure to environmental and lifestyle factors such as alcohol, obesity and environmental pollutants; endocrine disorders and hormonal imbalances; and aspects such as social pressure, late marriage and late childbirth. These have repercussions in psychological alterations, marital failure, risk of violence and social esteem, though in approximately 20–30% of cases of female infertility, it is not possible to know its etiology [57, 58, 59, 60, 61, 62].
Worldwide, infertility affects 48 million couples, which is equivalent to 15% of the world’s population. In Mexico, it is reported that 1.5 million couples suffer from infertility. More than 90% of fertility problems can be solved with assisted reproduction techniques [1, 6, 21, 57, 63, 64].
The high prevalence of infertility worldwide has affected 8–12% of couples of reproductive age, most of whom live in developing countries [59, 65]. Treatments for this condition include sex hormone therapy, surgical procedures such as tubal plastic surgery, and ART; however, they can cause unavoidable side effects, such as ovarian hyperstimulation syndrome (OHSS) and mental disorders related to hormone therapy (Fig. 1) [58].
The risk of ectopic pregnancy has been associated with assisted reproductive therapy. The first record of an ectopic pregnancy following in vitro fertilization treatment with embryo transfer (IVF-ET) was documented in 1976. Among spontaneous pregnancies, approximately 1 to 2% are ectopic, with the incidence increasing to between 2.1% and 8.6% of pregnancies following assisted reproductive techniques [3, 5, 6, 28, 63].
Conventional in vitro fertilization (IVF) treatment begins with controlled ovarian hyperstimulation with the objective of increasing the number of oocytes suitable for fertilization to obtain a greater number of embryos, in addition to improving the endometrium. Once extracted and inseminated, the resulting embryos are transferred through the cervix into the uterine cavity under ultrasound guidance. If embryos remain, they can be preserved by cryopreservation and used in subsequent cycles. In these freeze‒thaw or donor embryo transfer cycles, natural hormonal cycles or supplements are used that suppress the development of new follicles, creating an environment very similar to the physiological environment of spontaneous gestation. Approximately 25% of infertile women present ovulatory dysfunction, mostly associated with polycystic ovarian syndrome (PCOS), in which 75% of patients present infertility. Controlled ovarian stimulation is necessary for this process [3, 66, 67].
The rate of ectopic pregnancy in women with PCOS undergoing IVF can vary depending on different factors, such as the severity of the PCOS, the woman’s age, her medical history and other individual factors. However, it has been shown that women with PCOS undergoing IVF have a slightly but not significantly higher risk of ectopic pregnancy than women without PCOS undergoing the same treatment [68, 69].
Ovarian stimulation protocols all increase the risk of ectopic gestation. In addition to tubal factor infertility, the use of assisted hatching and intracytoplasmic sperm injection, fresh versus frozen embryo transfer, the day of embryo transfer, and the specific hormonal environment of ovarian stimulation are risk factors that have been identified to promote extrauterine pregnancy [1, 70].
One of the most widely used drugs is clomiphene citrate, which is considered the first-choice treatment for PCOS. It has an antiestrogenic action, overriding the “feedback” mechanism, causing an increase in the secretion of gonadotropin releasing hormone (GnRH), follicle-stimulating hormone (FSH) and luteinizing hormone (LH), thereby positively impacting follicular growth and ovulation. However, the pregnancy rate is relatively low, 15 to 40%, and ovulation is restored between 60% and 90% [67, 70].
The use of clomiphene citrate for ovarian stimulation is associated with an
increased risk of EP, with an incidence of 2.5% to 7.9% of ectopic pregnancies
[12, 13, 14, 63, 70, 71]. This is probably explained by the anti-estrogenic
phenomenon that negatively modifies the conditions for embryo implantation by
decreasing the thickness of the luteal endometrium with a reduction in the
concentrations of cytosolic estradiol and progesterone receptors and the
inhibition of epithelial proliferation due to the action of estradiol, making it
poorly receptive to embryo implantation, while in the fallopian tubes, it
potentiates aberrant apoptosis in the epithelium of the isthmic region at the
ciliary level, inactivating the estrogen receptor
Another drug with antiestrogenic capacity is tamoxifen, which has ovulation and gestation rates similar to clomiphene because it shares with this drug the selective action of nonsteroidal estrogen receptors [67, 76].
Aromatase inhibitors such as letrozole are used to stimulate ovulation, with the advantage of not causing adverse effects on the mucosa or endometrium, as it does not have anti-estrogenic action. In addition, it is associated with monofollicular development, reducing the risk of ovarian hyperstimulation; however, a greater association with congenital anomalies has been described, in addition to the incidence of ectopic pregnancy (EP), which has been reported in 6% of women who take aromatase inhibitors [67, 71].
Gonadotropins are the drugs of second choice when there is a failure in stimulation with selective nonsteroidal estrogenic modulators, and their function is to increase follicular development through the recruitment of antral follicles, increasing potentially fertile oocytes [14, 67, 71].
Other treatment alternatives used in ovulation induction are gonadotropin agonists and GnRH antagonists with direct pituitary action, which inhibit FSH and LH production. Their use is limited because their use is linked to very complex procedures such as in vitro fertilization. The rates of ectopic gestation induced by these drugs compared with those of the general population remain higher, and an incidence of 6 to 7.9% of ectopic gestations produced using gonadotropins has been described [14, 67, 71].
This treatment alternative was developed in Cambridge by the physiologist endocrinologist Robert Edwards and the obstetrician gynecologist Patrick Steptoe, who in 1976 achieved the first pregnancy through IVF. It had the disadvantage of being of ectopic location, and it was not until two years later that the first birth generated by this method was achieved. Thus, IVF is associated with an increased risk of EP, with a reported increase of up to 9.3 times with the use of this technique. With a reported rate between 1% and 5%, it is frequently located in the tubal, cervical, interstitial, and heterotopic regions [3, 5, 6, 18, 63, 66, 77].
An incidence of EP of 2.1 to 9.4% has been described in IVF techniques with intracytoplasmic sperm injection, while for IVF with embryo transfer, an incidence of 3.5 to 8.6% has been described, compared to 1.9% in natural pregnancies [18].
Physiologically, an egg that has been successfully fertilized initiates a division while descending the fallopian tube, then enters the uterine cavity approximately between Days 3 and 4 after fertilization, at which time it generates a structure called a blastocyst, characterized by having a cavity occupied by a liquid, which between Day 6 and 7 initiates an implantation in the epithelium of the internal mucosa of the uterine cavity. The contractility mechanism of the myometrium is based on the uterine waves generated after ovulation, which originate from the cervix and propagate toward the bottom of the cavity; this movement decreases during the luteal phase until it becomes almost inactive on Day 7 after the administration of hCG [1].
Thus, embryos that are transferred on Day 3 do not implant immediately, which can cause them to undergo retrograde transport into the fallopian tube through the retrograde contractions offered by the myometrial layer of the uterus, generating ectopic implantation. This time before embryo implantation is reduced if the transfer is performed on Day 5, together with the advantage that the diameter is greater in the blastocyst stage, which increases the resistance to contractile movement of the uterine cavity, which in this period is more inactive and with greater synchrony between the embryo and the receptor capacity of the endometrium. It is suggested to opt for the transfer of the blastocyst to the uterine cavity 7 days after the administration of the hCG hormone, since a better interaction with the endometrium has been seen than with the use of embryos in the cleavage stage because in this period, the contractions of the cervix to the fundus of the uterus are almost totally diminished [1, 6, 18, 66].
Genetic alterations are an important factor in implantation, since in natural cycles, aneuploidy can confer retrograde migration, in addition to the fact that abnormal trophoblasts have greater activity, leading to implantation at earlier stages, so extending the in vitro culture time to 5 days allows a better selection of chromosomally competent embryos, since aneuploidies do not develop until the blastocyst stage [1, 66].
The choice of the type of cycle to which the patient will undergo confers a greater or lesser risk of EP, since there is an association with lower rates of EP when performing IVF-ET in cycles that do not require ovarian stimulation such as freeze and thaw or donor cycles, decreasing the chances of extrauterine gestation by up to 65% compared to fresh cycles in which the tubal and uterine environment is modified, influenced by hormonal activity, causing an increase in uterine contractility and retrograde movement of the embryo toward the fallopian tube [1, 3, 18, 51, 66].
There is a dose-response relationship with the number of embryos transferred, since it has been demonstrated that fewer than three embryos counteracts the EP rate, a factor that is aggravated by the improvements that have been made in ART, which confer a better implantation potential, increasing the probability of extrauterine localization. The history of bilateral salpingectomy makes it necessary to reduce the number of embryos transferred and that this transfer can be of subfundal location, since the risk of EP increases proportionally to the depth and the amount of volume injected, as well as with the position of the patient [1, 3, 17, 51, 63].
Myosalpinx controls the salpingeal transit, in conjunction with the movement of the ciliated epithelium of the mucosa and the secretion of tubal fluid, mainly influenced by the hormonal balance between estradiol-17/S and progesterone, since an elevated level of estrogens is related to a decrease in the function of the estrogen receptor and modifications in the intensity, frequency and direction of the tubal peristalsis, generating a tubal blockage responsible for the arrest of the ovum in the salpingeum, as commonly occurs during ovarian stimulation cycles. Fallopian tube patency is an important factor involved in the retrograde movement of the embryo, since the risk of EP with only one permeable tube is 13.2%, compared with no patency or with both tubes permeable, which confer a risk of 2.9% and 4.4%, respectively [3, 18, 63, 78].
There is an increased risk regardless of the type of cycle chosen when the patient has a history of salpingoplasty, hydrotubation, infectious processes in the uterine cavity or adnexa, abdominal surgery, previous ectopic pregnancy, curettage or induced labor, as well as a history of smoking, a factor that when combined with a history of salpingectomy confers a higher risk of implantation in the callus or stump of the fallopian tube [1, 3, 17, 18, 51, 66, 78].
Advanced maternal age is associated with an increased risk of EP in fresh cycles without a donor. The highest incidence is in women aged between 35 and 44 years, mainly because aging that causes an accumulation of risk factors and anatomical and functional changes [1].
Assisted reproductive treatments offer the advantage of stricter control of the patient during the gestation period, which allows the timely and frequent identification of ectopic pregnancies compared to the findings that would be obtained in spontaneous pregnancies in which the diagnosis of this pathology may be delayed.
In patients with ectopic pregnancies, several areas are affected, such as the emotional burden and economic repercussions of pregnancy obtained through assisted reproductive techniques, together with a delay in the treatment itself, so it is essential to identify the main risk factors associated with these ARTs. The identification of the main risk factors associated with these ARTs is essential to modify them or, failing that, to opt for alternatives identified in these treatments to reduce the incidence of ectopic gestations and thus counteract the probability of maternal death, the most serious complication of this pathology, which is mainly associated with hypovolemic shock due to rupture of the ectopic pregnancy.
During the gestation period, it is of utmost importance to reinforce the education about and identification of the key symptoms that require medical attention to reduce the percentage of women who end their condition with surgical treatment and to thus counteract the increase in complications generated by these procedures, mainly impaired fertility.
The first-contact physician should be informed and identify risk factors that may be associated with an ectopic pregnancy, along with signs and symptoms such as pelvic pain and vaginal bleeding, to refer the patient for follow-up and favorable treatment in a timely manner.
ART, assisted reproduction techniques;
KDJO, MIO and GBR drafted the manuscript and conducted the search, study selection, and initial and final analysis. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
