- Academic Editor
Background: Considering the presence of an inflammatory process in the
pathogenesis of endometriosis, anti-inflammatory agents could be an alternative
option. The study aimed to elucidate the curative efficacy of Ursodeoxycholic
acid (UDCA) on the experimental rat model of endometriosis. Methods:
This experimental research included a total of 60 mature female Wistar albino
rats (250
Endometriosis, stroma outside the uterine-cavity, is a chronic condition characterized by the ectopic location of the functional endometrial gland [1]. It is the main reason of chronic pelvic related pains in premenopausal females [2]. In addition, it is one of the important causes of infertility in women, and due to its adhesive effect in oviducts, it also causes decreased ovarian reserve and embryo quality and implantation possibility [3]. Although many theories about the pathogenesis of endometriosis have been proposed, its origin is not fully clarified [4].
Cytokines and chemokines play an important role in its pathophysiology proving
that endometriosis is a chronic inflammatory process [5]. The increase of
macrophages causes the growth of ectopic endometrial lesions and angiogenesis
leading to chronic pain and infertility. Another evidence can be stated as the
demonstration of increased plasma cells and activated macrophages in
endometriotic lesions in immunohistochemical studies [6]. Agostinis et
al. [7] explored the anti-inflammatory and proapoptotic effect of this
combination on human endometriotic endothelial cells and mice treated with
N-acetylcysteine presented a lower number of cysts, smaller in size, compared to
untreated mice. As another molecule, Alpha-Lipoic acid is a natural antioxidant
synthetized by plants and animals, identified as a catalytic agent for oxidative
decarboxylation of pyruvate and
Ursodeoxycholic acid (UDCA) has attracted attention due to its effectiveness in the treatment of primary biliary cholangitis. UDCA has a cytoprotective effect in cholestatic liver disease with an anti-apoptotic mechanism as well as an anti-inflammatory effect due to its glucocorticoid receptor [10]. Apart from the liver and biliary tract diseases, UDCA showed a protective effect in inflammatory disease due to its anti-inflammatory effect. Considering the presence of an inflammatory process in the pathogenesis of endometriosis, the treatment with anti-inflammatory agents could be an alternative option for certain patients. The study aimed to investigate the curative efficacy of UDCA on the experimental rat model.
This animal trial was assessed and approved by the Animal Studies Ethical Board
of the Dumlupinar University (ID: 20151208-date:22/12/2022) and was compliant with
“Principles of laboratory animal care - 1985”. This research included a total
of 60 mature Wistar albino rats (weight range: 250
Anesthesia of all animals has been performed by administering ketaminehydrochloride (70 mg/kg, Ketas; Eczacibasi, Turkey), xylazinehydrochloride (7 mg/kg, Rompun: Bayer Company, Istanbul, Turkey) intraperitoneally under aseptic conditions. Skin antisepsis has been administered to all rats via 10% povidone-iodine solution during the operation time. While the rats, which were determined to be in the estrus stage, were taken into surgery, vaginal smear samples were examined with Papanicolaou.
The same researcher was interested in the surgical procedures applied to the rats in the study. A vertical incision was made in the lower midline region of the rats in a 5 cm wide abdominal laparotomy. An autologous part of uterine was located into the inner-surface of the right abdominal wall to achieve the experimental endometriosis procedure and thus surgically induced in rats. The endometrial part of the right uterus was transplanted into the right peritoneal cavity with the inner surface of the endometrium as opposed to the peritoneum and fixed on two sides. Then, 2 mL saline was injected into the abdominal-cavity to eliminate the possibility of drying on the abdominal wall and to prevent adhesion formation. The anterior abdominal wall was tapped as 2 layers using vicryl 3/0, prolene 4/0, respectively.
Endometrial implants were created in rats in the first laparotomy. Rats were found to
have successfully developed endometrial implant focus at the end of the four-week
waiting period were included in the study. 3 rats died after the first
laparotomy. A second laparotomy was applied to the rats to understand whether
endometriosis had occurred. In the second laparotomy approach, firstly,
peritoneal fluid samples were taken from all rats. While rats were found to have
endometriosis were included in the study, 5 rats without endometriotic focus were
excluded from the study. After measuring the volumes of endometriosis-developing
tissues as length, width and height, they were calculated according to the
formula (V (mm
After the second laparotomy, the rats were divided into two groups. While 11 rats were included in the study group, 11 rats were included in the control group. 150 mg/kg/day UDCA (Galenica SA, Athens, Greece) was dissolved in 1 mL sterile saline solution and administered orally for 40 days to the rats in the study group. In the control group, 1 mL of saline without active substance was administered orally for 40 days. During the period from the second laparotomy to the third laparotomy, a total of 2 rats, 1 rat from the study group and 1 rat from the control group, died. The study was continued with a total of 20 rats, 10 rats in the control and 10 rats in the UDCA. At the end of 40 days, the rats in both groups underwent a third laparotomy. During the third laparotomy, peritoneal fluid samples were taken from the rats and tissue samples were taken from the foci previously found to be endometriosis for histopathological examination, and the procedure was terminated. The rats were euthanized by giving a lethal dose of Ketamine after the procedure.
During the first, second, and third laparotomy, peritoneal fluid was obtained
and centrifuged at 200 g for 5 minutes and the supernatants were separated for
storage at –80 °C. The electrochemiluminescence immunoassay (ELISA) kits used in this study to measure tumor necrosis factor-
Tissue samples taken during the second and third laparotomy were fixed in a 10% neutral buffered formaldehyde solution. Then, the samples went through the dehydration stage and paraffin blocks were prepared from the tissue samples. 4-micron thick sections were prepared from paraffin blocks with the help of a microtome and stained with hemotoxylineosin. Samples were analyzed under a light microscope (Nikon Eclipse Ni light microscope was used in the study. Nikon DS-Ri2 imaging system on the same microscope was used to view histopathological specimens). In this study, histological assessment was based on visualization of the endometrial stroma and glandular. Scoring after the histopathological examination was made with the following criteria: 3: Well-preserved epithelial tissue, 2: Moderately preserved epithelial and leukocyte infiltration, 1: Small amount of epithelial cell, 0: Cell is not visible [11].
Data analysis was done using the SPSS v23.0 (IBM Corp., Armonk, NY, USA), the
Statistical program for Windows, while graph drawings were performed using the
Graph-Pad Prism Software v9.1 (GraphPad Software, Inc., San Diego, CA, USA).
Normality analysis was completed with the Kolmogorov-Smirnov test. For
comparisons of the groups, the “Independent Sample T-Test” was used
for the two groups, and the “Paired T-Test” compared the values before
& after the treatment. The Sample-T-Test was used to examine whether
there is a difference among MMP-2, TGF-
As given in Table 1 and Fig. 1, in the
comparison of the pre-treatment laparotomy and the standard groups, MMP-2,
TGF-
| Variables | Standard | Laparotomy (pre-treatment) | p-value |
| MMP-2, pg/mL | 0.705 |
0.968 |
0.120 |
| TGF- |
1.005 |
1.161 |
0.359 |
| TIMP-1, pg/mL | 1.043 |
0.920 |
0.455 |
| TNF- |
0.322 |
0.461 |
0.015 |
All data were given as Mean
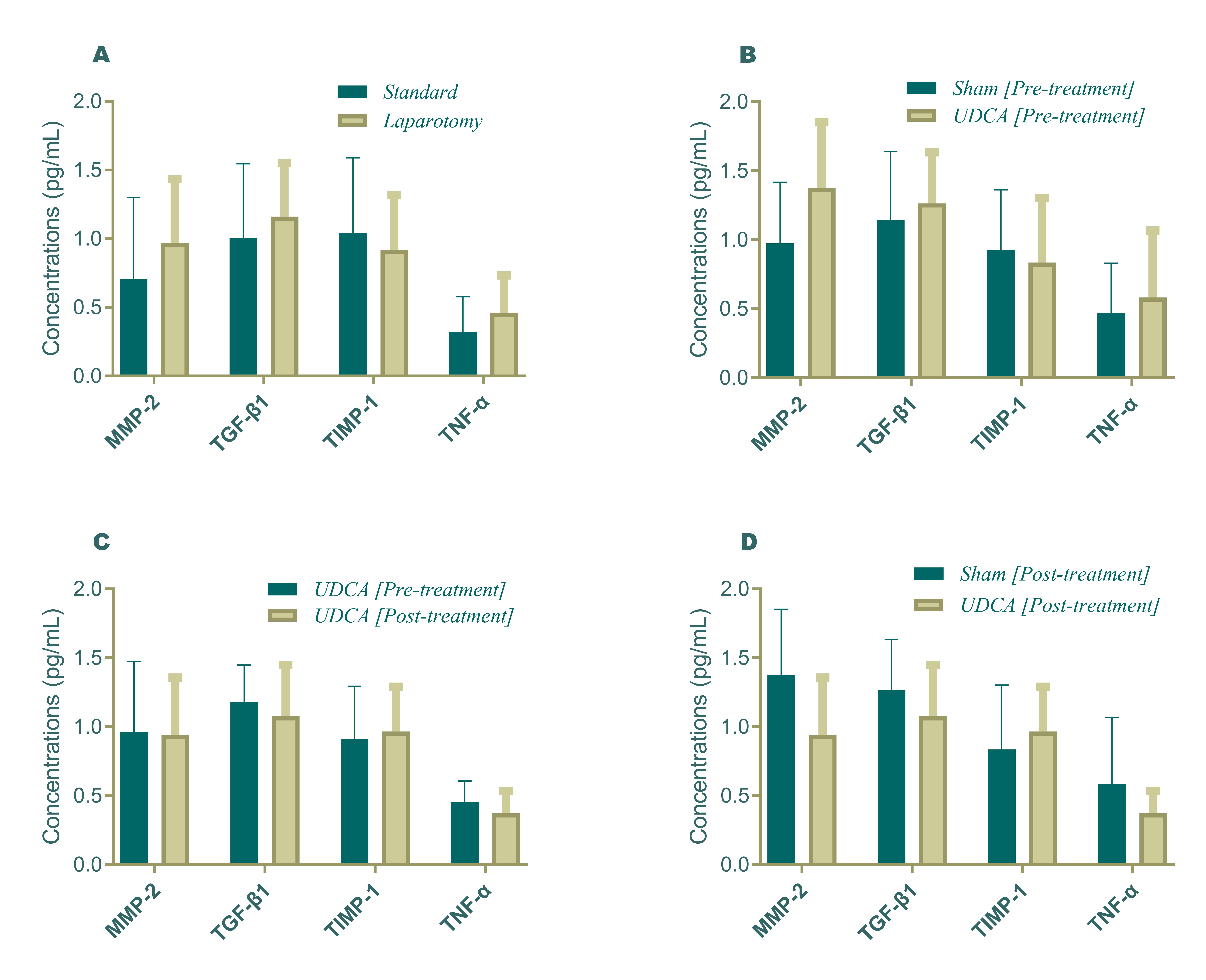 Fig. 1.
Fig. 1.Results of transforming growth factor
The data evaluation of the control and the UDCA-treated group before and after
the treatment are separately given in Tables 2,3. Accordingly, the MMP-2 value
after treatment (1.378
| Variables | Pre-treatment | Post-treatment (Sham) | p-value |
| MMP-2, pg/mL | 0.974 |
1.378 |
0.028 |
| TGF- |
1.145 |
1.265 |
0.180 |
| TIMP-1, pg/mL | 0.928 |
0.836 |
0.726 |
| TNF- |
0.470 |
0.582 |
0.611 |
| Endometriotic Volume (mm |
169 |
251 |
0.007 |
All data were given as Mean
| Variables | Pre-treatment | Post-treatment | p-value |
| MMP-2, pg/mL | 0.961 |
0.941 |
0.917 |
| TGF- |
1.177 |
1.076 |
0.752 |
| TIMP-1, pg/mL | 0.912 |
0.965 |
0.678 |
| TNF- |
0.452 |
0.372 |
0.109 |
| Endometriotic Volume (mm |
165.7 |
43.3 |
0.005 |
All data were given as Mean
When comparing the pre-treatment and post-treatment values of the UDCA group,
the post-treatment Endometriotic focal volume (43.3
| Variables | Sham | UDCA | p-value |
| MMP-2, pg/mL | 1.378 |
0.941 |
0.049 |
| TGF- |
1.265 |
1.076 |
0.290 |
| TIMP-1, pg/mL | 0.836 |
0.965 |
0.226 |
| TNF- |
0.582 |
0.372 |
0.705 |
| Endometriotic Volume (mm |
251 |
43.3 |
|
| Histology score | 2.6 |
1.0 |
0.001 |
All data were given as Mean
 Fig. 2.
Fig. 2.Endometriotic volume and histology scores in groups of Sham and Ursodeoxycholic acid (UDCA) rat models. (A) Comparisons of Endometriotic volume between the Sham post-treatment and UDCA post-reatment groups; (B) Comparisons of Histology scores between the Sham post-treatment and UDCA post-reatment groups.
In this study, we evaluated the effects of pre-treatment laparotomy and UDCA treatment on endometriosis-related markers and clinical outcomes. Our results show that the pre-treatment laparotomy group exhibited elevated TNF-
Endometriosis is a chronic inflammatory disease with unclear pathogenesis. It is an estrogen-dependent disease defined by the presence and growth of functional endometrial-like tissue, glands and stroma, outside the uterine cavity. A study by Laganà et al. [12] showed that macrophages are broadly classified into pro-inflammatory macrophages, which have selective anti-inflammatory and pro-fibrotic activities and are able to induce immunotolerance and angiogenesis. According to Ni et al. [13], the abnormal fecal metabolites, which are influenced by dysbacteriosis, may be the characteristics of Endometriosis mice and can be the potential important indices to distinguish the disease. Similarly, D’Alterio et al. [14] reported that endometriosis appears to be associated with elevated levels of different microorganisms across various microbiome. An ineffective immune response seems to play a role in its pathogenesis, and there is some scientific proof to state that the immune response may be modulated by the microbiome. Laboratory and clinical investigations indicate that hosts’ microbiome profiles with and without endometriosis can be different. After the initiation of endometriosis treatment, the symptoms can recur when the treatment is completed [15].
Endometriosis is defined as a chronic inflammatory disease, treatment with
anti-inflammatory agents appears to be prominent. TNF-
MMP-2 is in the matrix metalloproteinases enzyme group and this enzyme group plays a regulatory role in many physiological functions such as angiogenesis, inflammation, ovulation, embryogenesis [20]. The matrix metalloproteinase (MMP) enzyme group plays a role in extracellular matrix remodeling, which is associated with the proteolytic enzyme family, as well as in various pathologies such as tumor invasion [21]. Additionally, the high proteolytic activities of MMP enzymes play an important role in the pathogenesis of endometriosis [22]. In a study of endometriosis cases, MMP-2 was found to be high in peritoneal endometrial implants and it was thought that increased proteolytic activity affected pathogenesis [23]. Previously, it was determined that the level of MMP-2 increased in proportion with the severity of endometriosis as the disease progressed, more local and systemic MMP-2 levels increased, and more tissue remodeling has occurred, and MMP-2 increased invasion and progression further increased [24]. MMP-2 levels have been high in peritoneal fluid and serum of endometriosis patients. While the estrogen hormone increased MMP-2 on the other hand the progesterone hormone has prevented the development of endometriosis by decreasing MMP-2 [17]. Studies have revealed that MMP-2 was also associated with angiogenesis, tumor growth, invasion, and metastasis. MMP-2 has also been shown to play an important role in tumor growth, invasion, and metastasis in many types of cancer such as gastric cancer [25], lung cancer [26], and suppression of MMP2 is thought to be important for cancer treatment. In our study, MMP-2 following the treatment in the control was higher than before the treatment.
In our research, TIMP-1 has shown similarity in all of the groups. In the previous literature with endometriosis cases [27]. TIMP-1 levels decrease while MMP increase in breast cancer [28], on the contrary, there are also studies showing that TIMP-1 increases. In adenomyosis cases, MMP-2 and TIMP-1 was shown to increase together and it has determined that increase in TIMP-1 level developed secondary to MMP-2 increase, therefore preventing the invasive effect of MMP-2 [29]. The increase in TIMP-1 affects cell growth, angiogenesis, apoptosis, oncogenesis with a cytokine-like effect [30]. In a trial, the elevation of TIMP-1 was shown to indicate poor prognosis in colorectal cancer as a result of increased MMP in tumor invasion and metastasis, which had an effect of increasing tumor growth [31].
In our data evaluation, the endometriotic focal volume showed a signficant alteration by the treatment. Comparing the pre-treatment and posttreatment values of the UDCA group, the post-treatment endometriotic focal volume was significantly lower than the pre-treatment values.
As a minor limitation, we can notice the number of rats. Although a total of 60 mature female rats with no pregnancy were included in our research, it was the relatively low number of experimental animals in the present study.
As a result, the post-treatment endometriotic focal volume and histological
scoring decreased with UDCA. There was no significant change UDCA group before and after the treatment in terms of MMP-2, TGF-
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
AS, SKK, UE and AND designed the research study. AS and UE performed the research. SKK and AND provided help and advice on the experiments. UE analyzed the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This animal trial was assessed and approved by the Animal Studies Ethical Board of the Dumlupinar University (ID: 20151208/date: 22/12/2022) and is compliant with “Principles of laboratory animal care - 1985”.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
