- Academic Editor
Background: Coronavirus disease 2019 (COVID-19) has been found worldwide since its first outbreak in December 2019. Methods: This study investigated 347 pregnant women at approximately 39 weeks’ gestation from December 2022 to January 2023, which was divided into two groups: COVID-19 positive group (COVID-19) and COVID-19 negative group (Control). We analyzed blood parameters, liver function, and coagulation parameters of pregnant women with COVID-19 infection and in the Control group. Finally, we divided pregnant women with COVID-19 into two subgroups: No medication (n = 117) and Paracetamol treatment (n = 47), and analyzed effects of paracetamol treatment on the liver and blood coagulation function in COVID-19 infected pregnant women. Results: The alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum total bilirubin (TBIL), total bile acid (TBA), and lactate dehydrogenase (LDH) levels were significantly higher in pregnant women with COVID-19 than that of the control group. Elevated D-dimer, prolonged prothrombin time (PT), activated partial thromboplastin time (APTT), and low levels of fibrinogen (Fib) were observed in patients with COVID-19. There were no significant differences in the liver function between the drug treatment group and no medication group. Conclusions: COVID-19 caused abnormal liver function and blood coagulation function in pregnant women.
Since the end of December 2019, the world has been overwhelmed by coronavirus
disease 2019 (COVID-19) [1]. It is caused by a new type of
COVID-19 virus is susceptible to all populations, including pregnant women [2, 3]. Actually, pregnant women are more vulnerable to COVID-19 [4]. Compared to both non-pregnant and pregnant women who are not infected with COVID-19, pregnant women who are infected are at higher risk of developing serious medical conditions [5]. Studies have proved that COVID-19 increases pregnancy-associated complications and adverse pregnancy outcomes, including preeclampsia/eclampsia/hemolysis, elevated liver enzymes and low platelets (HELLP) syndrome, intensive care unit (ICU) occupancy rate, antibiotic use rate, premature delivery, and low birth weight [6]. COVID-19 during pregnancy is strongly associated with preeclampsia, especially among nulliparous women [7]. Newborns born to pregnant women infected with COVID-19 have a higher risk of morbidity, mortality, and neonatal intensive care unit (NICU) occupancy rate [6].
COVID-19 not only caused severe respiratory symptoms, but also led to a variety of extrapulmonary manifestations, such as thrombotic complications, acute liver and kidney injury, myocardial dysfunction and arrhythmia, thyroid dysfunction, and gastrointestinal symptoms [8]. The liver becomes the most frequently damaged organ besides the respiratory system. Chen et al. [9] firstly reported abnormal liver enzymes in patients with COVID-19. Their study revealed that serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) increased in 43.4% of COVID-19 patients. Most patients have slightly elevated transaminase, and only one patient has abnormally high transaminase level (ALT: 7590 U/L, AST: 1445 U/L). In another study [10], it was found that 39.1% (119/304) patients with COVID-19 presented elevated levels of ALT, AST, and total bilirubin (TBIL). Cai et al. [11] found that about 76% of COVID-19 infected people had abnormal liver transaminase and 21.5% suffered from liver damage during hospitalization. Meanwhile, blood coagulation abnormality occurred frequently in COVID-19 infected people. Patients with severe COVID-19 infection were more prone to suffer from COVID-19 related coagulopathy [12].
For pregnant woman, maintaining the optimal liver function and blood coagulation are beneficial to maternal health and fetal growth [13, 14]. Abnormal liver function and blood coagulation during pregnancy are common in many diseases, including preeclampsia, HELLP syndrome, intrahepatic cholestasis of pregnancy (ICP), acute fatty liver of pregnancy (AFLP), gestational diabetes or viral hepatitis. However, little is known about the effects of COVID-19 on the liver function and blood coagulation of pregnant women. The aim of our study is to study blood parameters, liver function, and coagulation parameters of pregnant women infected with COVID-19.
The information of participants was collected from December 2022 to January 2023. 347 pregnant patients were admitted and treated in the eastern district of Anhui Province Maternity & Child Health Hospital, Hefei, China. Excluding factors were as follows: gestational hypertension, preeclampsia, ICP, HELLP syndrome, AFLP, gestational diabetes, viral hepatitis, sepsis. We applied COVID-19 nucleic acid tests in all patients by nasopharyngeal swab, using reverse transcriptase polymerase chain reaction (RT-PCR), including 183 COVID-19-negative pregnant women (Control) and 164 COVID-19-positive pregnant women (COVID-19), as described in Fig. 1. Then, we divided the COVID-19 infected pregnant women group into two subgroups: No medication (n = 117) and Paracetamol treatment (n = 47), according to drug treatment (Fig. 1).
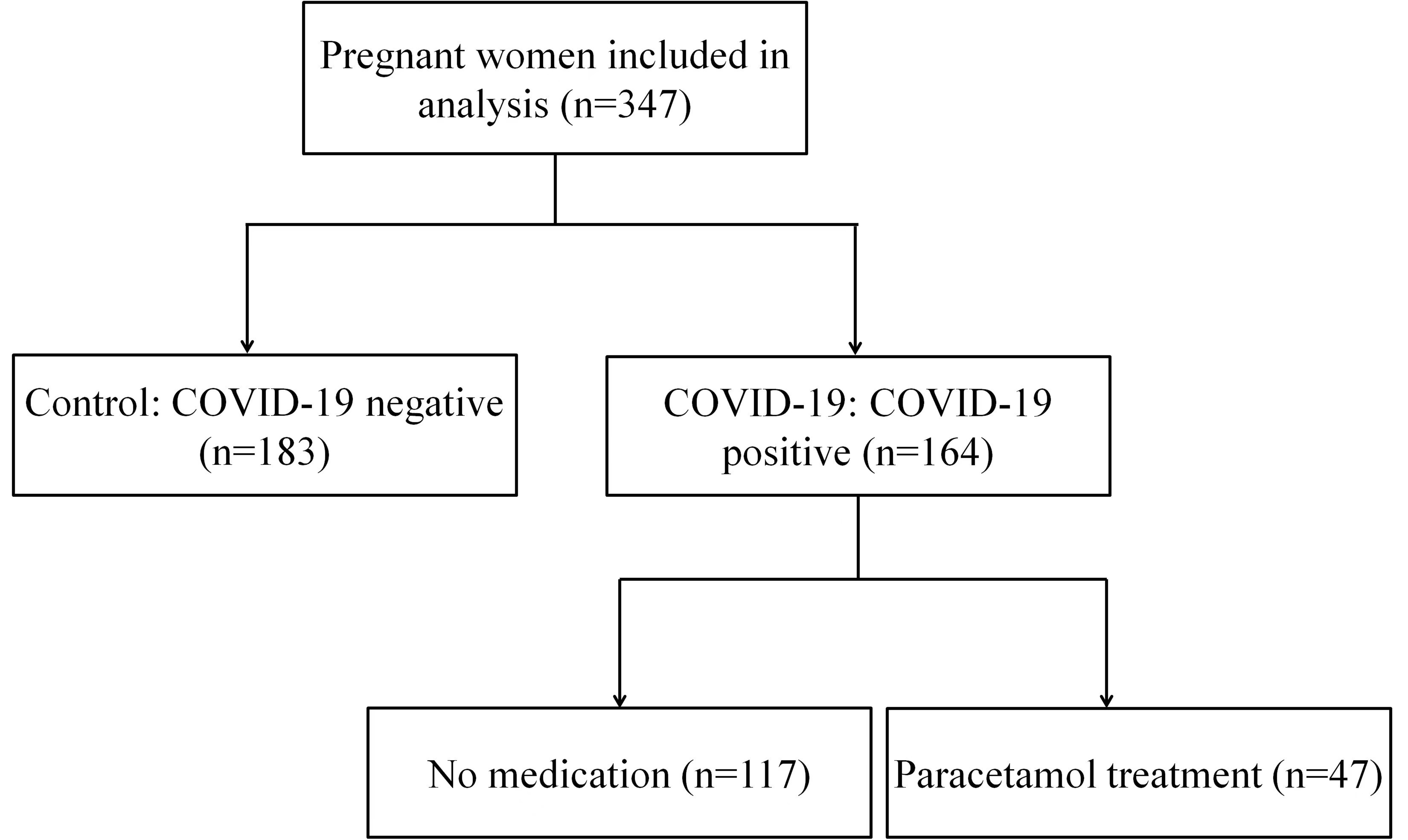 Fig. 1.
Fig. 1.Flow diagram for COVID-19-infected pregnant women.
The medical information of 347 pregnant patients was collected and examined by doctors from the obstetrics department. Laboratory characteristics were acquired by the hospitalization management system.
Serum samples were harvested from 347 pregnant women and all the laboratory data were obtained on the day of serum collection. Blood routine parameters including white blood cell (WBC), red blood cell (RBC), neutrophil count, lymphocyte count, neutrophil %, lymphocyte %, monocyte count, hemoglobin, monocyte %, platelet count, and C-reactive protein (CRP) were routinely measured by using standard methods.
Liver function was analyzed by TBIL, ALT, AST, alkaline phosphatase (ALP), LDH,
and total bile acid (TBA). Liver function abnormality was defined as the
elevation of either of the following liver enzymes in serum: ALT
Because pregnant women are a special population, antiviral drugs were not used in order to avoid any impact on the fetus. For 164 COVID-19 infected pregnant women, 117 COVID-19 were not treated with any drugs (No medication group), 47 COVID-19 were treated with paracetamol (H20093615, Jiangsu Hanchen Pharmaceutical Co., Ltd., Haimen, Jiangsu, China) according to their temperature and individual needs (Paracetamol treatment group).
The GraphPad Prism 8.0.2 software package (GraphPad Software, San Diego, CA,
USA) was used for statistical analyses. Data were presented as mean
In the study, 347 pregnant patients were admitted to our hospital, including 183 COVID-19-negative patients (Control) and 164 COVID-19-positive patients (COVID-19), as described in Fig. 1. Of the 164 pregnant women infected with COVID-19, 112 experienced a body temperature above 37.5 °C, and 47 of them took paracetamol orally to reduce their fever. The median age of pregnant women infected with COVID-19 was 29 years old, and the median gestational week of delivery was 39 weeks. Notably, one of the pregnant women did not undergo childbirth (Table 1). The clinical characteristics and information of the participants are summarized in Table 1.
| Variable | COVID-19 (n = 164) | Control (n = 183) | p-value | |
| Age (years) | 29 (18–44) | 29 (21–40) | 0.1021 | |
| Gestational week | 39 (29–41) | 39 (36–41) | 0.0069** | |
| Parity | 1 (0–3) | 1 (1–3) | ||
| Maternal BMI (kg/m |
27.06 (17.58–37.64) | 26.17 (22.10–35.11) | 0.08 | |
| Peripheral blood routine | ||||
| WBC count (10 |
7.25 (3.51–15.7) | 8.81 (4.66–14.12) | ||
| Neutrophil count (10 |
5.38 (2.16–13.51) | 6.18 (3.25–10.76) | ||
| Neutrophil % | 78 (50.5–91.3) | 74.5 (64.5–84.8) | ||
| Lymphocyte count (10 |
0.92 (0.2–4.85) | 1.53 (0.97–2.42) | ||
| Lymphocyte % | 14.05 (2.1–73.1) | 17.6 (11.5–28.3) | ||
| Monocyte count (10 |
0.49 (0.17–7.09) | 0.59 (0.28–1.18) | 0.005** | |
| Monocyte % | 7.4 (3.3–21.1) | 6.6 (3.3–13.1) | 0.003** | |
| RBC count (10 |
3.87 (1.16–5.57) | 3.94 (3.15–4.63) | 0.085 | |
| Hemoglobin (g/L) | 115 (77–141) | 120 (93–141) | ||
| Platelet count (10 |
164.5 (60–338) | 189 (124–407) | ||
| CRP (mg/L) | 8.27 (0.1–114.07) | 0.51 (0.1–34.28) | ||
| Coagulation function indicators | ||||
| D-dimer (µg/mL) | 1.52 (0.32–7.66) | 1.17 (0.43-11.7) | ||
| Fib (g/L) | 4.14 (2.09–7.84) | 4.27 (3.26–31.5) | 0.0123* | |
| PT (s) | 12 (9.9–14.1) | 11.5 (10.5–12.2) | ||
| APTT (s) | 35.3 (25.2–46.5) | 34.8 (28.2–40.2) | 0.0297* | |
| TT (s) | 15.3 (13.2–19.6) | 15.2 (13.6–17.1) | 0.6295 | |
| INR (%) | 0.93 (0.75–3.96) | 0.89 (0.81–0.97) | ||
| Liver function indicators | ||||
| TBIL (µM) | 8.955 (1.7–23.24) | 7.78 (4.92–14.91) | 0.0043** | |
| ALT (U/L) | 15.1 (2.5–502) | 9.2 (2.9–19.6) | ||
| AST (U/L) | 27.3 (9–353.9) | 15.7 (10.7–27.7) | ||
| ALP (U/L) | 144.3 (68.8–380) | 158 (99.2–689) | 0.0199* | |
| LDH (U/L) | 202.8 (39.5–812) | 192 (113.6–315) | 0.0042** | |
| TBA (µM) | 3.66 (0.38–42.4) | 2.3 (0.46–8.75) | ||
Note: Data are presented as median (range). BMI, body mass index; WBC, white
blood cell; RBC, red blood cell; CRP, C-reactive protein; Fib, fibrinogen; PT,
prothrombin time; APTT, activated partial thromboplastin time; TT, thrombin time;
INR, international normalized ratio; TBIL, total bilirubin; ALT, alanine
aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase;
LDH, lactate dehydrogenase; TBA, total bile acid. p-values comparing
COVID-19 and Control groups are from the Mann-Whitney U test. * p
Firstly, we analyzed peripheral blood parameters between COVID-19 and Control
groups. There was no significant difference for RBC between COVID-19 and Control
groups (Fig. 2A). Patients with COVID-19 had lower WBC count (7.24 vs
8.78
 Fig. 2.
Fig. 2.Blood routine parameters between COVID-19 and control groups.
(A) RBC, (B) WBC, (C) hemoglobin, (D) platelet count, (E) CRP, (F) lymphocyte
count, (G) lymphocyte %, (H) monocyte count, (I) monocyte %, (J) neutrophil
count, and (K) neutrophil %, comparing between COVID-19 and Control groups. Data
are presented as Mean
Liver function was assessed by measuring hepatocyte injury (AST and ALT), bile
duct injury or cholestasis (ALP). We also used markers of hepatic
clearance/biliary secretion capacity (TBIL and TBA). In this study, 14.63%
(24/164) of patients with COVID-19 presented elevated levels of ALT, and 26.22%
(43/164) of patients with COVID-19 showed elevated levels of AST. Only one
pregnant woman had abnormally high levels of Transaminase (ALT: 502 U/L, AST:
353.9 U/L), and her maximum temperature was 38.5 °C (Fig. 3A,B). The average ALT
and AST levels in the blood of pregnant women infected with COVID-19 were 31.2
U/L and 38.4 U/L respectively, which were two times higher than in the Control
group (Fig. 3A,B). ALP was decreased in patients with COVID-19 (Fig. 3C). Serum
TBIL and LDH levels were significantly higher in COVID-19 infected patients (Fig. 3D,F). Of the 164 pregnant women infected with COVID-19, 17 had TBA exceeding the
normal range (0‒10 µM), and the average TBA level was significantly
higher than that of the Control group (5.38 vs 2.85 µM;
p
 Fig. 3.
Fig. 3.Liver functions between COVID-19 and Control groups. (A) AST,
(B) ALT, (C) ALP, (D) TBIL, (E) TBA, and (F) LDH, comparing between COVID-19 and
Control groups. Data are presented as Mean
The liver plays a very crucial role in our body’s homeostasis. Liver injury
could increase the risk of hemorrhage or thrombosis by developing multiple
coagulation abnormalities, resulting from an imbalance between coagulation and
fibrinolysis. We analyzed the coagulation function by evaluating PT, APTT, INR,
and TT. A significant prolongation in PT (p
 Fig. 4.
Fig. 4.Blood coagulation parameters between COVID-19 and Control
groups. (A) PT, (B) APTT, (C) INR, (D) TT, (E) D-dimer, and (F) Fib, comparing
between COVID-19 and Control groups. Data are presented as Mean
Finally, we aimed to understand whether the paracetamol drug treatment affect liver function and blood coagulation function in COVID-19-infected pregnant people. In order to do that, we divided the COVID-19-positive pregnant women into two subgroups: No medication (n = 117, treated without any drugs) and Paracetamol treatment (n = 47, paracetamol treatment) according to drug treatment (Fig. 1). Indeed, 47 COVID-19 infected pregnant women with the symptom of fever were treated with paracetamol, according to their temperature. We found that there were no significant differences between paracetamol treatment and no medication group upon the liver function markers. However, we observed that the paracetamol treatment group had higher WBC, neutrophil count and lower lymphocyte count, and there were no significant differences in monocyte, RBC count, hemoglobin, platelet count and CRP concentration between the two groups. There were differences in PT, but they were all within the normal range (Table 2).
| Analytes | Paracetamol treatment (n = 47) | No medication (n = 117) | p-value | |
| Peripheral blood routine | ||||
| WBC count (10 |
7.94 (4.21‒12.41) | 6.88 (3.51‒15.7) | 0.0006*** | |
| Neutrophil count (10 |
6.67 (2.76‒11.29) | 5.06 (2.16‒13.51) | ||
| Neutrophil % | 80 (63.7‒91) | 76.7 (50.5‒91.3) | 0.0049** | |
| Lymphocyte count (10 |
0.71 (0.2‒4.85) | 0.99 (0.29‒2.52) | 0.0007*** | |
| Lymphocyte % | 7.9 (2.1‒73.1) | 15.9 (2.9‒36.2) | ||
| Monocyte count (10 |
0.5 (0.2‒7.09) | 0.48 (0.17‒1.76) | 0.3349 | |
| Monocyte % | 7.6 (3.4‒14.1) | 7.3 (3.3‒21.1) | 0.9444 | |
| RBC count (10 |
3.88 (1.16‒5.57) | 3.86 (2.88‒5.28) | 0.7962 | |
| Hemoglobin (g/L) | 115 (77‒138) | 115 (84‒141) | 0.9213 | |
| Platelet count (10 |
153 (75‒338) | 167 (60‒322) | 0.1170 | |
| CRP (mg/L) | 6.98 (0.2‒114.07) | 8.76 (0.1‒114.07) | 0.1526 | |
| Coagulation function | ||||
| D-dimer (µg/mL) | 1.63 (0.32‒7.66) | 1.51 (0.36‒6) | 0.8984 | |
| Fib (g/L) | 4.26 (2.09‒6.21) | 4.12 (2.6‒7.84) | 0.6316 | |
| PT (s) | 12.4 (10.4‒14) | 12 (9.9‒14.1) | 0.0157* | |
| APTT (s) | 35.4 (26.9‒40.7) | 35.2 (25.2‒46.5) | 0.7684 | |
| TT (s) | 15.3 (13.5‒17.4) | 15.3 (13.2‒19.6) | 0.6418 | |
| INR (%) | 0.97 (0.79‒3.96) | 0.91 (0.75‒1.09) | 0.0034 | |
| Liver function | ||||
| TBIL (µM) | 8.63 (4.78‒23.24) | 8.97 (1.7‒22.18) | 0.2625 | |
| ALT (U/L) | 13.8 (2.5‒105.8) | 15.3 (4.1‒502) | 0.3007 | |
| AST (U/L) | 27 (9.7‒115) | 28.5 (9‒353.9) | 0.6036 | |
| ALP (U/L) | 148 (74.8‒239) | 143 (68.8‒380) | 0.745 | |
| LDH (U/L) | 190 (39.5‒391) | 211.1 (138‒812) | 0.1823 | |
| TBA (µM) | 3.69 (1.03‒17.71) | 3.63 (0.38‒42.4) | 0.6048 | |
Note: Data are presented as median (range). * p
In the last three years, COVID-19 continued to spread worldwide, and putting significant strain on the global population. Even though most of COVID-19 positive patients were identified as mild, a minority of COVID-19 infected people had severe symptoms, such as respiratory failure, septic shock, or organ dysfunction [15]. COVID-19 is susceptible to all populations, including pregnant women. In fact, pregnant women with low immune function are more likely suffer from COVID-19. During pregnancy, due to high levels of estrogen and progestogen, the upper respiratory epithelium mucosa of pregnant women is swollen. This can make pregnant woman more prone to COVID-19 infection compared to the general population [16]. The COVID-19 pandemic has prompted the development of COVID-19 vaccine. As of February 2023, the global COVID-19 vaccination rate reached 65% [17]. Vaccination does not significantly increase the risk of side effects on mothers, fetuses, or newborns, which could also reduce the risk of natural and iatrogenic preterm labor during pregnancy [18].
As the largest solid organ in the body, the liver is responsible for detoxification and metabolism [19]. Recently, several studies reported the effect of COVID-19 on the liver function, which indicated that COVID-19 caused different levels of liver injury [11, 20, 21, 22]. Liver injury has been seen in many patients, particularly in those with serious illnesses. ALT and AST are markers of liver cell damage. ALT is a cytoplasmic enzyme that is abundant in the liver. AST is present in the cytoplasm and mitochondrial isoenzymes. The elevated levels of ALT and AST in the blood indicate the damage of liver cells. The values of serum AST, ALT, TBIL, TBA, and LDH were significantly higher in patients with COVID-19, indicating liver dysfunction in COVID-19-infected pregnant women [20]. As mentioned previously, an increasing number of people received COVID-19 vaccine. The question of whether the COVID-19 vaccine has a protective effect against liver damage remains a topic of concern. A previous study showed that the vaccinated population has lower levels of liver damage than that of unvaccinated population [17]. Given the liver is the processing hub for numerous circulating coagulation factors, liver injury is closely associated with the function of blood coagulation [23, 24, 25]. A report found that more than 85% patients with liver dysfunction suffer from abnormalities related to blood coagulation [26]. As a crucial molecular marker, the high levels of D-dimer indicate the hypercoagulable state and secondary hyperfibrinolysis [27]. As widely recognized, the level of D-dimer gradually increases during a normal pregnancy, reaching its peak value in the third trimester [28, 29, 30]. In our study, the level of D-dimer in COVID-19 positive pregnant women was higher than that in normal pregnant women. By comparing the blood coagulation function between severe and mild COVID-19 patients, Chen et al. [31] found that D-dimer were significantly higher in the critical COVID-19 patients, indicating that COVID-19 infection could cause coagulation dysfunction [32, 33]. As a classic coagulation parameter index, Fib is used to judge the bleeding tendency and hypercoagulable state of the body [13]. Extended PT and APTT are related to the severity of hepatic failure for bleeding risks and mortality [13]. In this study, prolonged PT, APTT, and low levels of Fib were observed in the COVID-19 infected pregnant women group, which means that COVID-19 infected pregnant women have hypercoagulability and bleeding tendency.
As a biochemical marker of acute inflammation, CRP is produced primarily in the
liver [34]. In our analyses of blood routine, liver function and blood
coagulation parameters, a significant increase of CRP was found in pregnant women
with COVID-19 infection than that of normal pregnant women (15.79 vs
2.84 mg/L, p
There are limitations in our study. Firstly, chronic diseases or maternal complications of analyzed pregnant women were not included in our study. As mentioned before, pregnant women are more prone to COVID-19 infections, and their symptoms and clinical characteristics can vary based on their internal chronic diseases or maternal complications. Secondly, diabetes and being overweight have been identified as high-risk factors for COVID-19 infection during pregnancy, and COVID-19 increases the risk of gestational diabetes [36]. The relationship between elevated liver enzymes in COVID-19 pregnant women and gestational diabetes requires further investigation. Thirdly, most of COVID-19 positive patients were identified as mild, we lack research on pregnant women with severe COVID-19. It is essential to conduct more in-depth follow-up studies to assess the health of both pregnant women and newborn babies.
In conclusion, COVID-19 can lead to abnormal blood parameters, liver function, and coagulation function. Dynamic monitoring of peripheral blood routine and liver functions is of great value in judging the progress and prognosis of COVID-19. Elevated D-dimer, prolonged PT, APTT, and low levels of Fib appeared in pregnant women with COVID-19 infection, which suggested that hypercoagulable state may play a role in the pathogenesis of COVID-19 infection, and anticoagulant therapy has potential benefits for COVID-19 patients.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
WS and HL designed the study. HL and JZ performed the research. HL provided help and advice on the ELISA experiments. WS analyzed the data and wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
This study was approved by Anhui Province Maternity & Child Health Hospital Review Board (No. YYLL2022–05-01), and all patients signed informed consent.
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflicts of interest statement.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
