- Academic Editor
Background: In this study, we sought to detect the expression of
complement C3 and C4 in serum and maternal-fetal interface in patients with
unexplained recurrent spontaneous abortion, and analyze their correlation, in
order to explore the clinical significance and role of C3 and C4 in unexplained
recurrent spontaneous abortion. Methods: In a prospective cohort study,
products of conception of 20 women who underwent curettage due to unexplained
recurrent spontaneous abortion in the Department of Obstetrics and Gynecology at
the Sichuan Provincial People’s Hospital from December 2021 to December 2022 were
chosen as the case group, and 23 healthy early-pregnancy women who underwent
elective abortion due to personal reasons during the same period were chosen as
the control group. Serum samples before curettage and decidual tissues samples
after curettage were collected. C3 and C4 levels in serum samples were detected
by immunoturbidimetry, and semi-quantitative scoring analysis was performed after
immunohistochemical staining of decidual tissues samples. The correlation between
C3 and C4 in serum and decidual tissues was analyzed. Results: The
levels of C3 and C4 in serum and decidual tissues in the case group were
significantly higher than those in the control group, and the differences were
statistically significant (p
Recurrent miscarriage, occurs between 1% and 5%, is a common complication in the field of women’s reproductive health. There is no uniform standard for the definition of unexplained recurrent spontaneous abortion (URSA) internationally. Most academics in China currently recommend defining recurrent spontaneous abortion (RSA) as 2 or more spontaneous abortions and recommend including biochemical pregnancies [1]. In approximately half of the patients with recurrent miscarriage there is no clear etiology, and these are referred to as unexplained recurrent spontaneous abortion (URSA) [2]. As the field of reproductive immunology continues to advance, more and more evidence points to a connection between the occurrence and progression of URSA and an imbalance in maternal-fetal immune tolerance [3]. The complement system is a crucial part of innate immunity, and recent research has demonstrated that it is also essential for adaptive immunity, among which C3 and C4 show the highest activity and content. Research has shown that C3 and C4 are involved in maintaining normal pregnancy through promoting placental development, embryo implantation, and supporting embryonic nutrition [4, 5]. However, excessive activation, genetic or acquired deficiencies, and dysregulation of complement C3 and C4 may contribute to adverse pregnancy outcomes such as recurrent spontaneous abortion (RSA), preeclampsia, and preterm birth through mediating amplified inflammatory responses and enhancing procoagulant effects. Due to the complexity and intricacy of the complement system, compared to other immune systems, research on the role of C3 and C4 in the etiology of RSA is relatively limited. Therefore, this study aims to explore the clinical significance and role of C3 and C4 in the imbalance of maternal-fetal immune tolerance in URSA patients by analyzing the differences and correlations in the expression levels of C3 and C4 in serum and decidual tissues.
This prospective cohort study enrolled patients underwent curettage surgery in the Department of Obstetrics and Gynecology of Sichuan Provincial People’s Hospital between December 2021 and December 2022. The study has been approved by Reproductive Medicine Ethics Committee of Sichuan Provincial People’s Hospital (approval number: 202105) and informed consent was obtained from the research subjects. Following strict inclusion and exclusion criteria, a total of 20 patients were included in URSA group, and 23 patients were included in the control group.
Inclusion criteria for the case group (URSA group) were as follows: two or more consecutive spontaneous abortions, including previous biochemical pregnancies. The current pregnancy is a natural pregnancy with no primitive cardiac pulsation detected by ultrasound, and the gestational age is before 13 weeks. Inclusion criteria for the control group were as follows: no history of spontaneous abortion, stillbirth, or fetal death, with at least one history of live birth. The current pregnancy is before 13 weeks of gestation with normal embryonic development detected by ultrasound, and the termination of pregnancy is requested for personal reasons. No abdominal pain, vaginal bleeding, or discomfort in early pregnancy.
Exclusion criteria were as follows: abnormal chromosomal karyotype analysis of the fetus or both partners; uterine anatomical abnormalities such as uterine malformation or intrauterine adhesion; endocrine diseases such as diabetes and hypothyroidism; reproductive tract infections such as Chlamydia and Mycoplasma; coagulation abnormalities; autoimmune diseases such as antiphospholipid antibody syndrome(APS), and systemic lupus erythematosus; a family history of hereditary diseases or history of malignant tumors.
Complement C3 assay kits and Complement C4 assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China). Complement C3 antibody and C4 antibody were purchased from the Proteintech company (Rosemont, IL, USA), and secondary antibody was purchased from the Servicebio company (Wuhan, Hubei, China). Digital trinocular camera microscopy imaging system was purchased from Meike Audi Industrial Group Co., Ltd. (Xiamen, Fujian, China), and the data image analysis system was purchased from Indica Labs company (Albuquerque, NM, USA).
5 mL of fasting venous blood from the cubital vein of patients undergoing curettage in the early morning before surgery was collected into an ethylenediamine tetra-acetic acid (EDTA) tube from the Becton, Dickinson and Company (Franklin Lakes, NJ, USA) . Blood specimens were centrifuged at 3000 rpm for 10 minutes in a low-temperature centrifuge, and the supernatant was carefully extracted and placed in Eppendorf (EP) cryovials from the Biosharp company (Hefei, Anhui, China), then rapidly frozen and stored in a small liquid nitrogen container and transported to a –80 °C ultra-low temperature freezer for storage until use. After curettage or artificial abortion, the decidua decidual tissues from the patients were taken, cleaned with phosphate buffered saline (PBS) to eliminate blood clots, fixed in 4% paraformaldehyde, embedded in paraffin, and stained with immunohistochemistry.
The serum levels of C3 and C4 were detected using the immunoturbidimetry according to the instructions of the reagent kit.
Paraffin-embedded sections were deparaffinized, antigen retrieved, endogenous
peroxidase blocked with 3% hydrogen peroxide, and serum blocked. After
incubation with primary antibodies (C3 or C4) and washing with PBS for 3 times,
secondary antibodies were added for incubation. Then, 3,3
All experimental data in this study were analyzed using SPSS 26.0 statistical
analysis software (IBM Corp., Armonk, NY, USA). Continuous data were expressed as
Statistical analysis was performed on the general clinical data of the URSA group and the control group. Age, body mass index (BMI), parity, and gestational weeks did not statistically differ between the two groups, demonstrating data comparability, as shown in Table 1.
| Baseline characteristics | URSA group (n = 20) | Control group (n = 23) | t/Z | p |
| Age (years) | 30.60 |
28.70 |
t = –1.527 | 0.135 |
| BMI (Kg/m |
20.94 |
21.05 |
t = 0.176 | 0.861 |
| Parity (times) | 2.75 |
2.48 |
Z = –1.177 | 0.239 |
| Gestational weeks (days) | 66.85 |
64.57 |
t = –1.422 | 0.163 |
BMI, Body Mass Index; URSA, unexplained recurrent spontaneous abortion. Mann-Whitney U test was used for parity, and t-test was used for other variables in the table.
The level of C3 in serum in the URSA group were significantly higher than those in the control group, (p = 0.015). The level of C4 in serum in the URSA group were also significantly higher than those in the control group, (p = 0.002) (Table 2).
| Group | URSA group (n = 20) | Control group (n = 23) | Z | p |
| Serum C3 (g/L) | 2.11 |
1.47 |
–2.423 | 0.015 |
| Serum C4 (g/L) | 0.23 |
0.13 |
–3.068 | 0.002 |
Mann-Whitney U test was used for both variables in the table.
The average positive scores of C3 in decidual tissues of the URSA group were significantly higher than those in the control group (p = 0.020). The average positive scores of C4 in decidual tissues of the URSA group were also significantly higher than those in the control group (p = 0.001). Positive scores were derived from the intensity and extent of positive C3 and C4 deposition at the maternal-fetal interface. In the positive group, complement C3 and C4 were found to be abundantly expressed in the cell membrane and cytoplasm of syncytiotrophoblasts, cytotrophoblasts, and mesenchymal cells, but in the negative group, only a small amount of C3 and C4 was found to be expressed in the cell membrane and cytoplasm of syncytiotrophoblasts, and no expression was found in mesenchymal cells. Refer to Table 3, Figs. 1,2 for details.
| Group | Average positive score of C3 | Average positive score of C4 |
| URSA group (n = 20) | 6.45 |
5.15 |
| Control group (n = 23) | 3.74 |
2.39 |
| Z | –2.330 | –3.445 |
| p | 0.020 | 0.001 |
Mann-Whitney U test was used for both variables in the table.
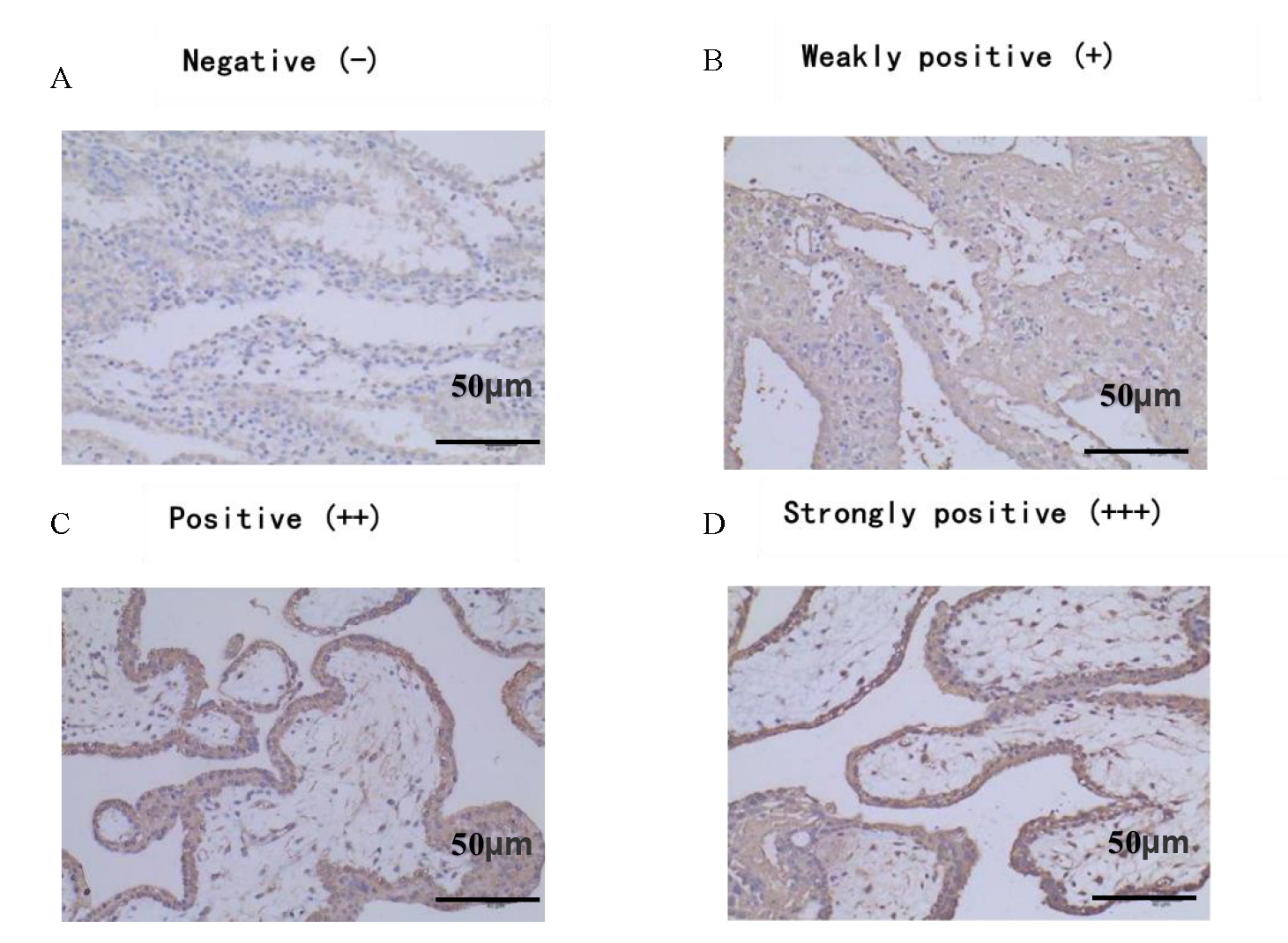 Fig. 1.
Fig. 1.Positive expression of C3 in decidual tissue
(Immunohistochemistry (IHC)
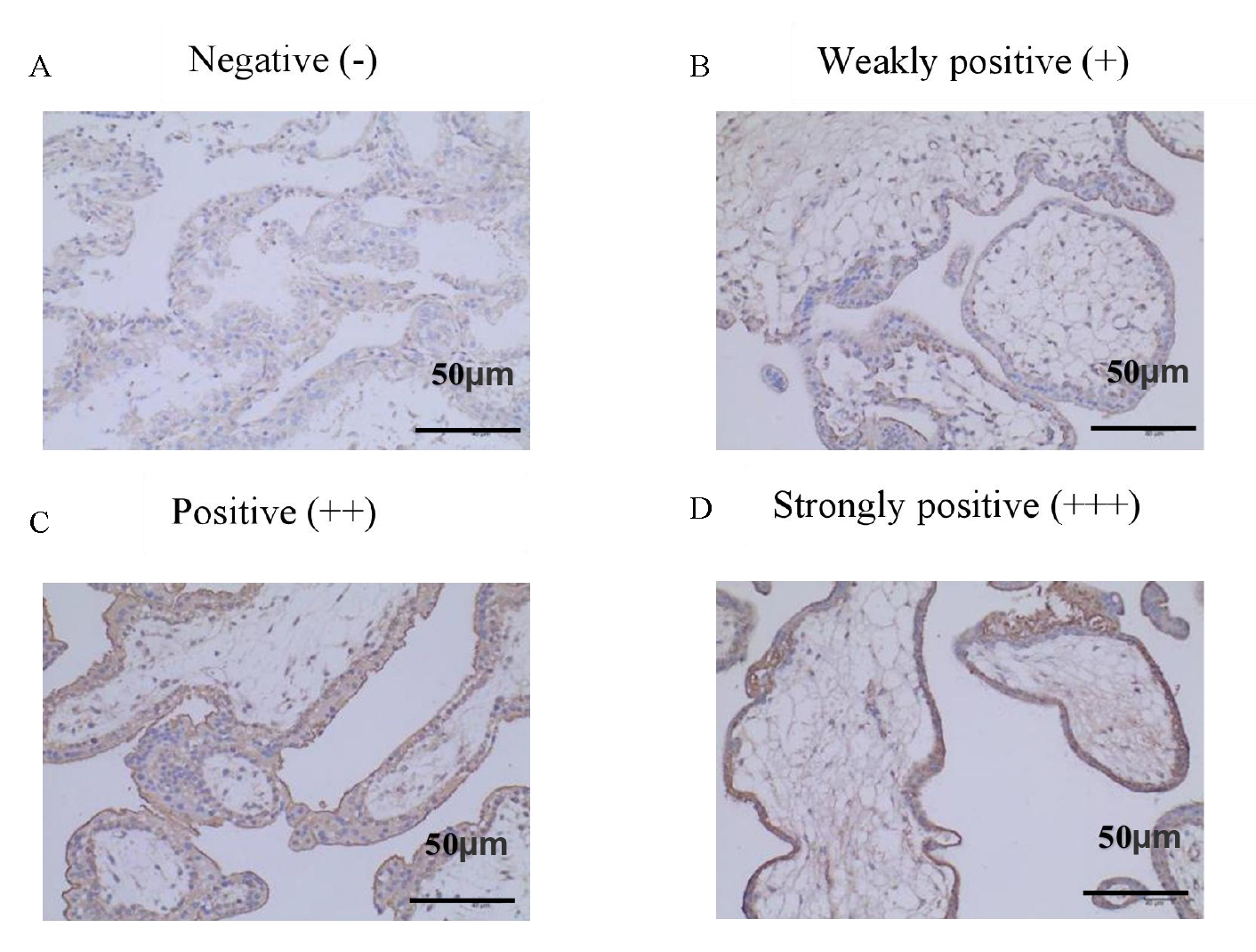 Fig. 2.
Fig. 2.Positive expression of C4 in decidual tissue (IHC
There was a significant and positive correlation between C3 in serum and C4 in serum of URSA patients (r = 0.481, p = 0.032) (Fig. 3), while there was no significant correlation before C3 and C4 in the maternal-fetal interface of URSA patients (r = –0.038, p = 0.873). Refer to Figs. 3,4 for details (Fig. 4).
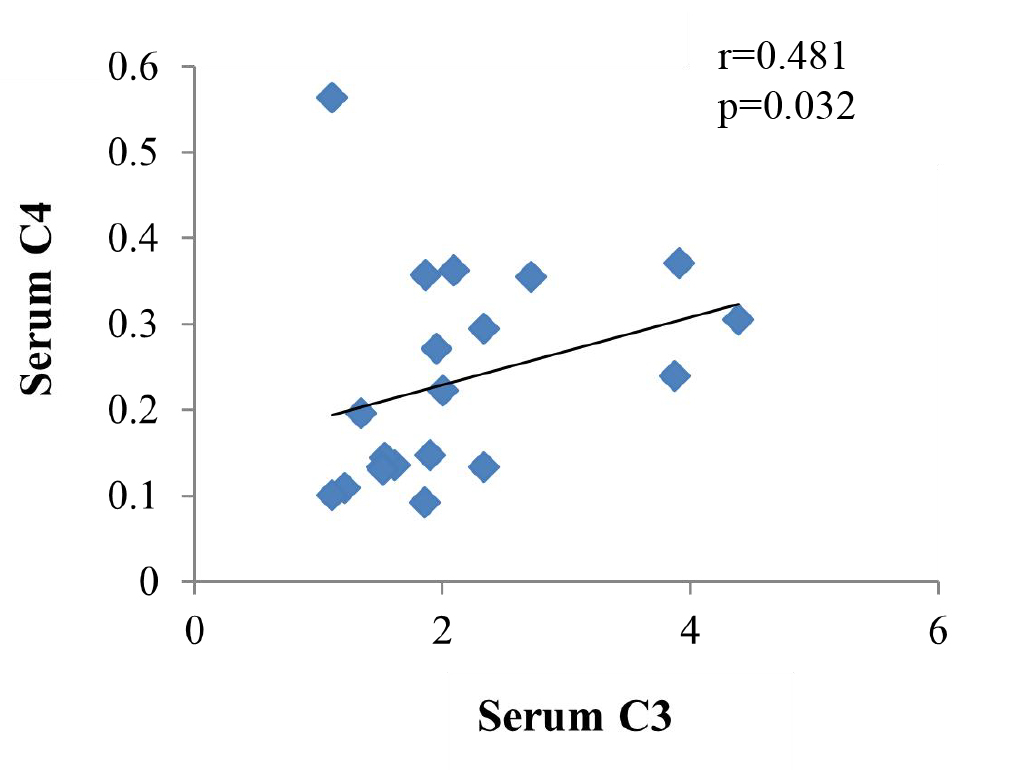 Fig. 3.
Fig. 3.Correlation between C3 and C4 in serum of URSA patients.
 Fig. 4.
Fig. 4.Correlation between C3 and C4 in the maternal-fetal interface of URSA patients.
There was no significant correlation between C3 in the serum of URSA patients and C3 levels in the maternal-fetal interface (r = –0.004, p = 0.986); there was no significant correlation between C4 in the serum of URSA patients and C4 levels in the maternal-fetal interface (r = –0.161, p = 0.499). Refer to Figs. 5,6 for details.
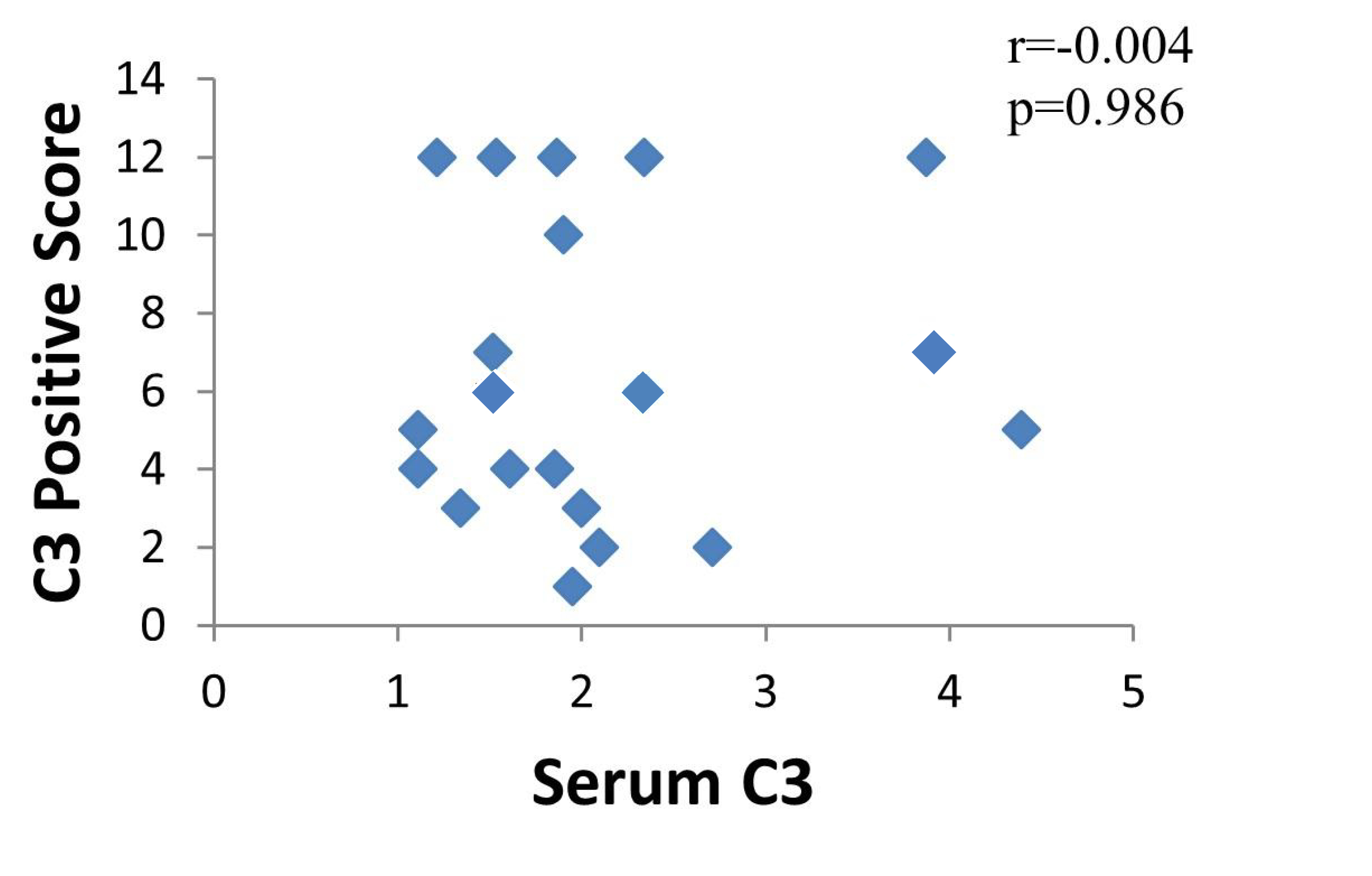 Fig. 5.
Fig. 5.Correlation between C3 in serum and C3 in the maternal-fetal interface of URSA patients.
 Fig. 6.
Fig. 6.Correlation between C4 in serum and C4 in the maternal-fetal interface of URSA patients.
The diagnosis, treatment and prevention of URSA is important in reproductive health. The results of this study showed that the development of URSA may be linked to excessively activated complement C3 and C4 levels; since they were found to be higher in the serum and decidual villi tissues of URSA patients than in the group of healthy early pregnant women. This is consistent with the results of previous studies on antibody-independent RSA models [4, 6]. Meuleman and coworkers observed C4 levels at the maternal-fetal interface; however, they did not study C3 levels in serum and decidual tissues [4].
The liver produces the majority of complement proteins, but other organs, such as the placenta, can also produce them locally [7]. In this study, certain levels of C3 and C4 were detected in the serum of normal healthy early pregnant women. Deposition of C3 and C4 was also detected in the trophoblast tissue of some patients, suggesting that complement activation is beneficial for the normal development of embryos during normal pregnancy, and the activation level of complement is within a certain range under normal pregnancies. This may be related to complement regulatory proteins at the maternal-fetal interface, including decay-accelerating factor (DAF, CD55), membrane cofactor protein (MCP, CD46), and recombinant cluster of differentiation 59 (CD59) [8]. Complement levels, however, cannot yet be compared with other studies since they differ between gestational weeks [8, 9].
In humans, DAF, MCP and CD59 are complement regulators in normal placenta [9]. DAF and MCP can control the activation of C3 in the early stage of the complement cascade reaction, while CD59 acts in the terminal pathway to prevent the formation of membrane attack complex (MAC). Their combined action inhibits excessive activation of complement, thereby avoiding loss of embryos due to complement attack [10]. When complement regulation is imbalanced, it can often lead to a number of adverse outcomes during pregnancy [7, 11]. The mRNA expressions of CD46 and CD55 decreased in the decidua taken by Banadakoppa et al. [12] from patients who had spontaneous abortions. Therefore, in our study, the elevated C3 and C4 levels at the maternal-fetal interface of URSA may also be due to dysregulation of complement regulatory proteins. However, we only performed positive deposition assays, and further study on the molecular pathways related to complement regulatory proteins is needed.
APS is an autoimmune disease characterized by the presence of persistent antiphospholipid antibodies (aPL). It is characterized by arterial and venous thrombosis and/or recurrent miscarriage and pregnancy complications [13], and it is one of the main causes of immune-related RSA. In APS patients, the excessive activation of the complement classical pathway or direct activation of the complement alternative pathway by immune complexes formed by a large amount of antigen-antibody complexes in the body can clear foreign substances, resulting in a depletion of plasma complement levels [13, 14]. In order to prevent bias from the negative effects of autoantibodies on pregnancy in our study, individuals with autoimmune illnesses were removed from the inclusion criteria in the case group. This was done to rule out autoantibody-mediated embryonic disorders such as aPL.
Complement levels are variable between gestational weeks [10, 15, 16]. He et al. [15] found that C3 levels were similar to that in non-pregnant women in early pregnancy and C4 levels began to rise in early pregnancy. Since our test only measured complement levels in the first trimester of pregnancy, complement levels at different gestational weeks cannot currently be compared. Although prior research has indicated that C4 levels are increased during early pregnancy, we still found significantly elevated C4 and C3 blood levels in URSA when compared to controls throughout this time period with no difference in gestational age. The results of this study suggest that there are abnormal complement levels in URSA patients, and excessive activation of complement in serum may be involved in the occurrence and development of miscarriage. However, the high expression levels of complement may be in the early stage of the immune response and therefore not consumed to low levels.
This study also found that the levels of C3 and C4 positive deposition at the maternal-fetal interface were significantly higher than those in normal healthy early pregnant women, suggesting excessive activation of C3 and C4 may be one of the immunological mechanisms leading to URSA. However, the causality between the increased levels and embryonic loss is not yet clear, whether it is due to excessive complement deposition in target tissues such as embryos and placenta causing an immune attack resulting in miscarriage, or due to complement activation induced by necrotic embryonic tissue enhancing the immune response and clearance of tissue debris clearance.
In this study, through correlation analysis of complement C3 and C4, it was found that there was a positive correlation between serum levels of C3 and C4. C3 is a common hub of the three complement activation pathways and the initiating activator of the complement alternative pathway [17], which is considered to be the most critical functional site in the complement system. C4 is an important component of the classical activation pathway, and an increase in its activity and degradation product C4 is considered to be a marker of the classical complement activation pathway [18]. As a result, this suggests that diverse complement activation cascade events occur in URSA patients, such as the classical pathway as well as the alternative pathway, and that various complement activation pathways may be involved in miscarriage. This also indicates the common characteristics and close relationship of complement activation pathways. Further research is needed on the molecular mechanisms of specific pathways, such as early activation factor C1q in the classical activation pathway, regulatory factors B factor and D factor in the alternative pathway, and mannose-binding lectin-associated serine protease (MASP) in the mannose-binding lectin (MBL) pathway.
There has been no correlation between complement in serum and decidual tissues in previous studies. Unfortunately, the results of this experiment suggest that C3 in serum did not correlate significantly with C3 at the maternal-fetal interface, and also with C4, indicating that the level of complement in serum may not be able to predict the level of complement at the maternal-fetal interface. This may be due to the small sample size in this study, which needs to be confirmed with a larger sample size, or it may be because there is an asynchrony between complement levels at the maternal-fetal interface and circulating complement levels, which will need to be confirmed by additional basic research.
Due to the complexity of the components and enzymatic cascades involved in the complement system, research on the specific mechanisms of complement abnormal activation pathways, key active substances, and adverse pregnancy outcomes is limited and controversial. The major limitation of our study is that we only measured C3 and C4 at the serum and maternal-fetal interface, but did not perform specific mechanistic studies. In addition, this was a single-center, small sample size study and future studies with larger samples sizes are needed.
In summary, C3 and C4 levels are elevated in the serum and maternal-fetal interface of patients with URSA, suggesting that abnormal complement activation may be one of the immunological mechanisms underlying URSA. C3 and C4 could serve as early diagnostic criteria for recurrent miscarriage. Furthermore, different complement activation pathways may collectively contribute to the occurrence of pregnancy loss, providing new insights for predicting miscarriage using complement. The development of tailored immunotherapies using complement and a reduction in the incidence of unfavorable pregnancy outcomes, will be facilitated by elucidating the precise processes underlying the link between complement and URSA, in future basic science research.
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
ZXZ—Principal Investigator (responsible for the project design, acquisition of data, analysis and interpretation of data, drafting the manuscript); HXX—Associate Investigator (contribution to data collection and obtain ethics approval); ML—Associate Investigator (contribution to data acquisition process and revision of the manuscript); RPL—Associate Investigator (contribution to data acquisition process); WJJ—Associate Investigator (contribution to data acquisition process); YZ and QL—Coordinating Principal Investigator (contribution to conception and design of the study, revision of the manuscript and final approval of the version to be published). All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was approved by Reproductive Medicine Ethics Committee of Sichuan Provincial People’s Hospital (approval number: 202105). We confirmed that informed consent was obtained from all patients and their families. We confirmed all methods were carried out in accordance with Helsinki declaration.
We acknowledge all the enrolled patients for their contribution to the study.
This study was funded by 2020 Chengdu University of Traditional Chinese Medicine’s Xinglin Scholar Talent Research Enhancement Plan Hospital Special Project (NO.2020yky04) and 2022 Project approval table of Sichuan Province Maternal and Child Medicine Science and Technology Innovation Project (NO.22FXZD02).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
