- Academic Editor
Background: Possible therapeutic benefits of lymphadenectomy (LND) in the treatment of endometrial cancer (EC) remain controversial. The present study was undertaken with the aim of investigating the prognostic role of LND in women with clinically confirmed, low-grade, uterus-confined endometrioid EC exhibiting lymphovascular space invasion. Methods: A bicentric retrospective review was conducted for the identification of cases of EC, treated at two gynecologic oncology departments in Turkey. Subsequently, the data of 1811 patients with EC (non-endometrioid, endometrioid, or mixed histology) who had undergone surgery between 2007 and 2016 were analyzed. After extracting data, 37 patients were defined as the study group, and those 37 cases were matched to 74 control patients who had undergone surgery with systematic LND to compare survival. Kaplan-Meier analysis was applied in the process of interpreting data on survival, and variables predicting patient outcomes were identified using Cox proportional hazards regression. Results: Five-year disease-free survival (DFS) rates were 88.2% versus 81.5% (p = 0.985), while overall survival (OS) rates were 91.0% versus 85.7% (p = 0.814) for the study and control groups, respectively. Advanced ages (hazard ratio (HR): 6.69; 95% confidence interval (CI): 1.59–28.09, p = 0.009) and tumors of grade 2 (HR: 3.35; 95% CI: 1.09–10.26, p = 0.034) were found to be independently predictive of decreased OS within the entire cohort. Conclusions: Systematic LND does not have a therapeutic role in the management of low-grade, uterus-confined endometrioid EC with lymphovascular space invasion. There was no difference between the survival outcomes of the two groups considered in this study.
The staging of cases of endometrial cancer (EC) is performed with the guidance of the International Federation of Gynecology and Obstetrics (FIGO). In the year 2021, following the specific update of risk factors, EC staging system was revised and introduced in 2023 [1, 2]. Lymphadenectomy (LND) remains a mandatory part of this staging since 2009 [1, 3]. While the updated staging system of EC suggests that sentinel lymph node (SLN) biopsy is a viable substitute for systematic LND in terms of staging, it is challenging for clinicians worldwide to access this new method uniformly.
Questions about the role of LND in EC have raised controversy. Although lymph node (LN) involvement changes the staging and guides adjuvant therapy, some studies, including Cochrane Reviews, have shown that LND has no therapeutic efficacy in early-stage EC [4, 5, 6].
Lymphovascular space invasion (LVSI) of the uterus is an important risk factor in cases of EC [7, 8]. LVSI is described as one of the most powerful independent predictors of pelvic LN metastasis; however, unfortunately, information about this predictive variable is not available to clinicians before surgery [9]. Although various authors have already defined risk scoring systems for both LN metastasis and LVSI positivity, there is currently no definitive method that conclusively demonstrates these associations [10, 11].
The 2016 guideline published by the European Society of Gynecologic Oncology (ESGO) classified cases of LVSI-positive grade 1–2 EC in the high-intermediate risk group, while the adjuvant therapy management options of the high-intermediate risk group were specified as either “nodal staging performed” or “nodal staging not performed” [12]. In addition, the recent revised staging system for EC classified substantial LVSI positive cases as stage IIB [2].
Although the study groups of Benedetti Panici et al. [4] and the ASTEC (A study in treatment of Endometrial Cancer) trial did not demonstrate any improvements in disease-free survival (DFS) or overall survival (OS) among patients who had undergone LND, both of those randomized trials had important limitations, including the extent of LND and lack of information about LVSI status [4, 6]. Although LVSI has been previously described as a good predictive variable for LN metastasis [13], the prognostic role of systematic LND in LVSI-positive low-grade clinically confirmed early-stage EC remains unclear. Although two retrospective series showed worse survival outcomes in cases of LVSI-positive early-stage EC, the prognostic role of LND was not analyzed [14, 15]. The present retrospective study, conducted in two institutions, aimed to assess the prognostic role of LND in cases of clinically confirmed, low-grade, LVSI-positive early-stage endometrioid EC.
The patient files of consecutive women who underwent primary surgery for EC, between January 2007 and December 2016, during their treatment at one of two selected Turkish gynecologic oncology centers were retrieved and subjected to retrospective reviews. Approval of the research protocol was granted by the relevant local institutional review board (Approval Number: 10). Furthermore, all patients included in this study had signed informed consent forms before their surgery, allowing the inclusion of their medical data in future scientific research’s.
The cases included in the study group comprised patients clinically diagnosed with pure endometrioid-type EC that was confined to the uterus; these patients underwent surgery without systematic LND. Patients were deemed eligible for inclusion in the study group if LVSI positivity was noted in the final pathology report. The following cases were excluded from the study group: women who had non-endometrioid type EC, cases of mixed histology, patients without confirmed LVSI positivity, cases of concomitant macroscopic extrauterine tumors with any disease activity found during visual inspections of the abdominal cavity and pelvic region, and all patients with incomplete medical records. Also excluded from this group were patients with grade 3 tumors and involvement of the stroma of the cervix. In addition, patients with identified synchronous malignancies and individuals with enlarged nodes in the preoperative scanning were excluded.
Each of the selected cases described above was matched to two patients
clinically diagnosed with uterine-confined endometrioid-type EC who had undergone
surgical procedures that incorporated systematic LND in the same period. The
definition of adequate systematic LND was taken as a minimum of 15 removed pelvic
LNs and a minimum of 5 removed paraaortic LNs (PALN) [16]. Matching of the
patients in the two considered groups was based upon consideration of age at time
of diagnosis and year of the diagnosis (for both criteria,
Surgical specimens from all patients enrolled in the study were examined by
gynecological pathologists. In the course of their interpretations, tumors were
described based on information from initial pathology reports with relevant
subsequent data, including grades of the tumors, myometrial invasion depths
categorized as either
For all cases, attending physicians or multidisciplinary tumor boards at the two considered institutions made all relevant treatment decisions. Postoperative management was finalized in light of the histological findings reflecting patients’ general conditions, findings from the surgical specimens, and the ages of the patients. Adjuvant radiotherapy comprised external beam radiation therapy, including or not including vaginal brachytherapy, and external beam radiation therapy was administered as intensity-modulated radiotherapy or via image-guidance methods. Adjuvant chemotherapy usually included multiple agents, with carboplatin plus paclitaxel being the most often used regimen. Decisions concerning adjuvant treatments were not standardized, and these approaches varied between the two considered centers.
Patients returned for their follow-up evaluations every 3 months for the first 2 years, every 6 months for the subsequent 3 years, and then once a year thereafter. In these follow-up evaluations, magnetic resonance imaging or computerized tomography was performed annually. The last calculation of survival data, as presented in this study, was performed on September 1, 2022. Patients were evaluated as alive or dead at the last follow-up for evaluations of survival data. In this process, to confirm all recorded deaths of the enrolled patients, the national social security death index was regularly reviewed.
Following the initial diagnoses of all patients, cases of recurrence were identified based on evidence of metastases obtained via appropriate imaging techniques and physical examinations. The definition of DFS was taken as the period extending from primary surgical procedures to the first observation of recurrence based on radiologic imaging findings and serum carbohydrate antigen 125 (CA125) measurements or death due to any cause, or date of final contact for those who had remained alive without recurrence at the end of the follow-up duration. Any elevation in CA125 levels was always assessed in conjunction with imaging methods. Calculations of OS were based on the durations between primary surgeries and dates of death or final contact. Patients who remained alive at the end of the research were censored in the survival analyses at the date of the last known follow-up.
Survival analyses were conducted in line with the Kaplan-Meier method, and the
results were subsequently compared employing log-rank testing. For statistical
analysis, chi-square and Student t-tests were applied as appropriate for
unpaired data. IBM SPSS Statistics 26.0 (IBM Corp., Armonk, NY, USA) was used in
all statistical processes described here. The threshold for statistical
significance was taken as p
We identified 1811 women treated for EC during the period considered here. Among that initial population, 398 of the patients were positive for LVSI, and 304 of those 398 patients had pure endometrioid-type EC. After careful exclusion of macroscopic extrauterine tumors, incomplete medical records, grade 3 tumors, and patients with cervical stromal involvement, 37 patients were defined as the study group. These 37 women were matched with 74 control cases comprising patients who had undergone surgery with systematic LND. All relevant demographic and clinical characteristics of the cases reviewed in this study are presented in Table 1.
| Characteristics | Study group | Control group | p-value | |
| (n = 37) | (n = 74) | |||
| Median age at surgery, years (range) | 59 (34–83) | 62 (40–85) | 0.054 | |
| Menopausal status, n (%) | 0.437 | |||
| Premenopausal | 4 (10.8%) | 4 (5.4%) | ||
| Postmenopausal | 33 (89.2%) | 70 (94.6%) | ||
| Myometrial invasion |
13 (35.1%) | 57 (77.0%) | ||
| Grade, n (%) | 0.836 | |||
| Grade 1 | 13 (35.1%) | 29 (39.2%) | ||
| Grade 2 | 24 (64.9%) | 45 (60.8%) | ||
| Median tumor size, cm (range) | ||||
| 4 (10.8%) | 1 (1.4%) | 0.024 | ||
| 27 (73.0%) | 30 (40.5%) | |||
| Serum CA125, IU/mL | 0.831 | |||
| Normal ( |
26 (70.3%) | 50 (67.6%) | ||
| High ( |
11 (29.7%) | 24 (32.4%) | ||
| Positive peritoneal cytology, n (%) | 2 (5.6%) | 5 (6.8%) | 0.809 | |
| Median number of LNs removed, n (range) | - | 53.50 (24–139) | NA | |
| Number of pelvic LNs removed | 35 (14–78) | |||
| Number of PALNs removed | 16 (5–61) | |||
| LN metastasis, n (%) | - | 25 (33.8) | NA | |
| Pelvic LN metastasis only | 12 (48.0%) | |||
| PALN metastasis only | 2 (8.0%) | |||
| Pelvic and PALN metastases | 11 (44.0%) | |||
| Stage, n (%) | NA | |||
| IA | 24 (64.9%) | 13 (17.6%) | ||
| IB | 13 (35.1%) | 36 (48.6%) | ||
| IIIC1 | - | 12 (16.2%) | ||
| IIIC2 | - | 13 (17.6%) | ||
| Adjuvant Treatment | NA | |||
| No additional treatment | - | 8 (10.8%) | ||
| Brachytherapy only | 12 (32.4%) | 14 (18.9%) | ||
| EBRT | 25 (67.6%) | 23 (31.1%) | ||
| Chemo-radiation | - | 22 (29.7%) | ||
| EBRT + Brachytherapy | - | 7 (9.5%) | ||
| Follow-up, months (range) | 47 | 55 | 0.769 | |
| Recurrence, n (%) | 6 (16.2%) | 13 (17.5%) | 0.999 | |
| Status, n (%) | 0.771 | |||
| Alive | 33 (89.2%) | 64 (86.5%) | ||
| Dead | 4 (10.8%) | 10 (13.5%) | ||
Abbreviations: EBRT, external beam radiation therapy; LN, lymph node; LND, lymphadenectomy; NA, not applicable; PALN, paraaortic lymph nodes; CA125, carbohydrate antigen 125.
Age, menopausal status, tumor grade, baseline value of serum CA125, positive
peritoneal cytology, recurrence rates, and median duration of follow-up did not
differ significantly between the two groups. However, the patients in the control
group were statistically more likely to have had myometrial invasions extending
beyond the considered depth threshold of 50%: 77.0% of tumors in control group
had depths of
The control group of this study was designed to include only cases treated by pelvic and PALN. Among these cases, a total median of 53.5 LNs were removed (range: 24–139). Respectively, the median numbers of removed pelvic LNs and PALNs were 35 (range: 14–78) and 16 (range: 5–61). Furthermore, in the control group, 25 of 74 patients (33.8%) were found to have experienced LN metastasis. Metastasis to pelvic sites, paraaortic sites, and both were respectively noted in 12, 2, and 11 cases. In the study group, 24 (64.9%) cases were classed as stage IA, and 13 (35.1%) cases as stage IB. In the control group, on the other hand, 13 (17.6%) cases were classed as stage IA, 36 (48.6%) cases as stage IB, 12 (16.2%) cases as stage IIIC1, and 13 (17.6%) cases as stage IIIC2.
Among the entire cohort considered (n = 111), 8 (7.2%) women did not undergo any additional treatment after surgery. On the other hand, adjuvant treatment entailed brachytherapy for 26 (23.4%) patients, while 7 (6.3%) received external beam radiotherapy in conjunction with brachytherapy postoperatively. 48 (43.2%) patients underwent external beam radiotherapy alone, whereas 22 (19.8%) were treated with chemoradiation. A total of 19 cases of recurrence were observed (17.1%). Specifically, these cases included 10 (9.0%) cases of loco-regional recurrence, 7 (6.3%) cases of retroperitoneal failure, and 2 (1.8%) cases of distant relapse.
Upon consideration of the data obtained for 5-year DFS, the findings were seen to be statistically similar between the study and control groups, at rates of 88.2% and 81.5%, respectively (p = 0.985). Likewise, the OS rates for the duration of the study did not differ with statistical significance between the study and control groups, at rates of 91.0% and 85.7%, respectively (p = 0.814). The DFS and OS curves are depicted in Figs. 1,2, respectively.
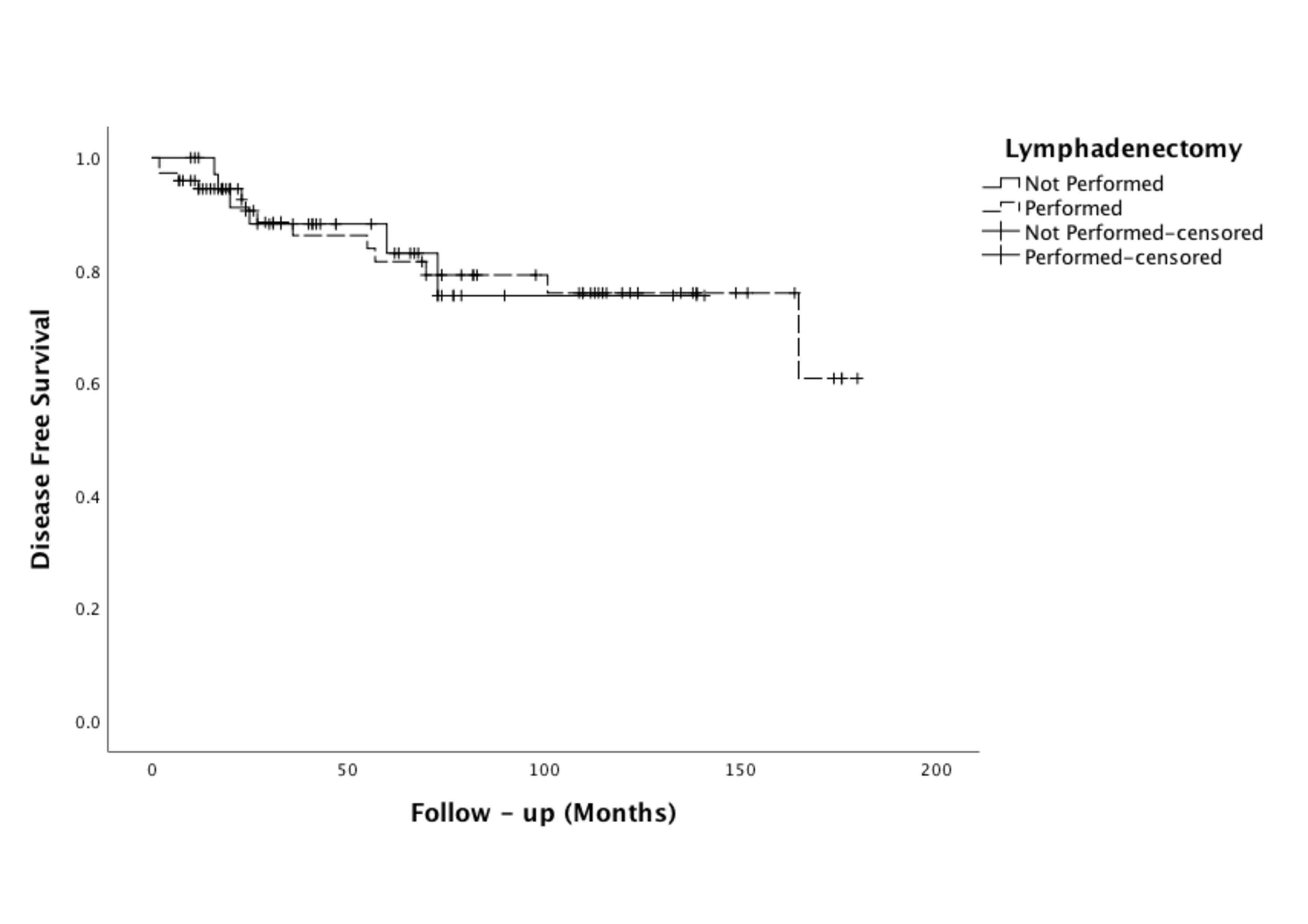 Fig. 1.
Fig. 1.DFS curve of women whether LND not performed (n = 37) or performed (n = 74). DFS, disease-free survival; LND, lymphadenectomy.
 Fig. 2.
Fig. 2.OS curve of women whether LND not performed (n = 37) or performed (n = 74). OS, overall survival; LND, lymphadenectomy.
Neither univariate nor multivariate analysis revealed any significant factors for shorter durations of DFS among the entire cohort of patients (Table 2). On the other hand, upon review of the results of multivariate analysis for OS, it was concluded that advanced age (HR: 6.69, 95% CI: 1.59–28.09, p = 0.009) and tumors of grade 2 (HR: 3.35, 95% CI: 1.09–10.26, p = 0.034) independently predicted the outcome of decreased OS, as presented in Table 3. Of the 37 women in the study group, 4 (10.8%) were dead and 33 (89.2%) were alive at the end of the study. The corresponding numbers were respectively 10 (13.5%) and 64 (86.5%) among the controls (Table 1).
| Prognostic factors | Univariate analysis | Multivariate analysis | |||
| p-value | HR | 95% CI | p-value | ||
| Age ( |
0.415 | ||||
| Menopausal status (Premenopausal vs. Postmenopausal) | 0.724 | ||||
| Myometrial invasion ( |
0.728 | ||||
| Grade (1 vs. 2) | 0.077 | 2.15 | 0.85–5.42. | 0.103 | |
| Tumor size, cm | |||||
| ( |
0.539 | ||||
| ( |
0.336 | ||||
| CA125, IU/mL ( |
0.253 | 1.57 | 0.63–3.95 | 0.330 | |
| Cytology (positive vs. negative) | 0.641 | ||||
| LND (Performed vs. Not performed) | 0.985 | ||||
Abbreviations: HR, hazard ratio; 95% CI, 95% confidence interval; DFS, disease-free survival; LND, lymphadenectomy.
| Prognostic factors | Univariate analysis | Multivariate analysis | |||
| p-value | HR | 95% CI | p-value | ||
| Age ( |
0.069 | 6.69 | 1.59–28.09 | 0.009 | |
| Menopausal status (Premenopausal vs. Postmenopausal) | 0.288 | ||||
| Myometrial invasion ( |
0.595 | ||||
| Grade (1 vs. 2) | 0.140 | 3.35 | 1.09–10.26 | 0.034 | |
| Tumor size, cm | 2.82 | 0.90–8.84 | 0.074 | ||
| ( |
0.427 | ||||
| ( |
0.088 | ||||
| CA125, IU/mL ( |
0.102 | 3.08 | 1.00–9.48 | 0.050 | |
| Cytology (positive vs. negative) | 0.934 | ||||
| LND (Performed vs. Not performed) | 0.814 | ||||
Abbreviations: OS, overall survival; LND, lymphadenectomy.
The present study was planned with the aim of assessing possible impacts of systemic LND, on survival outcomes among women with clinical diagnoses of low-grade LVSI-positive uterine-confined EC. Our findings revealed no association between systematic LND, and extended DFS or OS among the considered cases. However, certain limitations of this study need to be noted. The main limitations of this study are its retrospective design and the absence of central pathology reviews. Without central pathology reviews, we were unable to utilize the three-tiered scoring system for LVSI [18]. Furthermore, impacts of specific adjuvant treatments on survival could not be assessed. The increased frequency of adjuvant treatments applications introduces a statistical bias in efforts to understand the survival rates of women with EC undergoing LND. In light of the retrospective design and the dual-institutional nature of this study, we must acknowledge limitations in our ability to provide detailed indications of adjuvant treatments, including brachytherapy, external beam radiotherapy, and chemotherapy.
The application of systematic LND remains a longstanding debate in the
management of EC. Two retrospective series demonstrated therapeutic benefits of
systematic LND for intermediate-risk and high-risk patients [19, 20]. Eggemann
et al. [19] investigated 1502 cases of EC, and subgroup analysis was
performed for 690 patients with intermediate risk. Accordingly, significant
survival benefits were observed among the patients who underwent systematic LND.
Multivariate analysis also revealed that LND of the pelvic region alone (HR:
0.63, 95% CI: 0.38–0.82, p = 0.001) and LND of both the pelvic and
paraaortic regions (HR: 0.50, 95% CI: 0.43–0.81, p
In another large retrospective series, Todo et al. [20] investigated the therapeutic role of pelvic and PALN. As in our study, they classified patients according to uterine risk factors, and a survival advantage was found in the group of cases of intermediate risk. However, in that study, all LVSI-positive stage I patients were classified in the same group, irrespective of grade. Grade is a uterine risk factor that is commonly known before surgery. Moreover, most high-grade EC patients will have undergone pelvic and PALN during their surgeries. Low-grade cases, on the other hand, are a major problem because LVSI positivity unknown before surgery may be an accompanying factor after hysterectomy, and this will move the patient into the high-risk group according to current guidelines. Our study sought a solution to this problem and we showed that systematic LND has no benefits for survival among low-grade cases of high-to-intermediate risk.
Benedetti Panici et al. [4] and ASTEC study group et al. [6] conducted randomized controlled trials to assess the impacts of LND on survival and nearly 2000 patients were included in their works. Although their findings suggested that LND was not capable of improving DFS or OS, they were unable to perform subgroup analysis or document LVSI status. Considering these limitations of previous prospective studies, we designed the present research in a retrospective manner to explore the impact of LND on low-grade LVSI-positive EC, and we did not find any survival benefits. Furthermore, every patient in our control group underwent LND up to the left renal vein. In comparison to our study, Benedetti Panici et al. [4] performed PALN for 26% of patients in their study population.
Besides the two aforementioned prospective randomized clinical trials, various retrospective research studies have considered the possible therapeutic benefits of LND [21, 22, 23, 24, 25]. Although impacts on survival rates were generally found to be most remarkable among cases of intermediate-risk and high-risk EC, all of the studies cited here had important limitations. For instance, Smith et al. [25] and Cragun et al. [23] did not specify the LVSI statuses of the patients. Huang et al. [24] did not evaluate the survival of the patients according to uterine risk factors. Chan et al. [22] evaluated the impact of the extent of LN resection on survival rates of women with intermediate-risk or high-risk cases of endometrioid uterine cancer. However, they provided no documentation of LVSI status. Although Bassarak et al. [21] showed survival benefits among both high-grade and low-grade cases of endometrioid adenocarcinoma, they compared LND outcomes to cases treated without LND, without specifying any details about the specific types of LND procedures.
Several authors have demonstrated that tumor size and myometrial invasion may influence prognosis, especially in cases of low-risk disease [26, 27]. Despite all patients in our study being LVSI-positive and categorized as high-tontermediate risk, the study group, which did not undergo LND, exhibited lower tumor size and myometrial invasion. Although this situation may initially appear to introduce bias into our study’s results, the Cox regression analysis indicated that these two factors did not impact prognosis.
Adjuvant therapy options vary across different institutions for patients with early-stage EC who exhibit LVSI positivity [28]. Moreover, the latest guidelines indicate that cases with positive LVSI differ in terms of adjuvant treatment selection, depending on whether nodal staging is performed or not [12, 29]. In our study, variations are also observed in the application of adjuvant treatments. Due to the limited number of patients, our study has been insufficient in determining the effectiveness of adjuvant treatment. Although it could be inferred that performing LND in the study group might prevent unnecessary administration of adjuvant treatment, there are studies demonstrating that LVSI positivity alone is an independent prognostic factor [9, 14].
In recent years, the concept of SLN assessment, utilized in place of systematic LND, has gained significance in the surgical staging of EC. The latest ESGO guideline has specifically acknowledged its potential applicability and utilization [29]. Although sentinel node biopsy has become the most prevalent mode of LN evaluation, it should be emphasized that the presence of LVSI has been reported to be an independent risk factor for failed SLN mapping (odds ratio (OR): 0.126, 95% CI: 0.24–0.658) in women with EC [30]. Up to 29% of women with positive LVSI status have failed bilateral mapping [31]. While the SLN concept replaces the utilization of systematic LND in cases of failed bilateral mapping, evaluating the survival outcomes of these patients remains crucial. Currently, there are two ongoing randomized controlled trials that are investigating the prognostic importance of nodal staging in early-stage EC [32, 33]. We believe that our study will be particularly beneficial in regions with low socioeconomic status and limited access to SLN capabilities.
In addition to the importance of SLN concept, the latest ESGO guideline and the revised FIGO 2023 staging emphasize the significance of molecular analysis on EC prognosis [2, 29]. Current staging system still segregate cases with LN involvement (stage III) from stages I/II for defining treatment options. While early-stage cases with p53 mutations are classified as high-risk, the significance of this marker’s positivity in advanced stages remains uncertain. Since not all clinicians globally will have equal access to the utilization of molecular markers, challenges in the practical implementation of the FIGO 2023 staging are expected to endure for an indefinite duration.
All studies described here also had serious limitations pertaining to patient selection and the extent of LND. The main advantage of our study is that all patients in the control group underwent systematic LND up to the left renal vessel, providing more consistency among the obtained findings. In addition, patients with high-to-intermediate risk were considered, according to the ESGO guidelines, which call for adjuvant therapy regardless of whether LND was performed for the patient or not. A comparison of the groups in terms of OS according to, whether LND was performed or not, allowed for more reliable comparative findings. This is another major advantage of our study. In the future, we recommend that guidelines for principal adjuvant treatment choices be identical for all cases of high-to-intermediate risk, regardless of the systematic LND status of the patients.
While the therapeutic efficacy of LND remains a topic of debate, there is a consensus against its direct therapeutic role. Nevertheless, the importance of accurately identifying nodal involvement cannot be underestimated. This identification is essential for guiding the appropriate administration of adjuvant therapy, aiming to strike a balance that prevents both inadequate treatment and excessive intervention.
In conclusion, our analysis revealed similar durations of DFS and OS between patients for whom LND was performed and those for whom it was not. Although our study has limitations that include the lack of a standardized adjuvant treatment protocol, we acknowledge that integrating the recent ESGO/ European Society for Radiotherapy & Oncology (ESTRO) risk classification into our analysis could have provided further clarity regarding the role of LND in early-stage EC. Nevertheless, our findings may assist gynecologic oncology surgeons in identifying the necessary extent of surgery in endometrioid-type cases of EC. However, these findings need to be further validated by prospective research, focusing on adjuvant treatment modalities in all relevant subgroups of early-stage endometrioid-type EC.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
İY, KB and ÖC designed the research study. BE and ZFC provided the idea for the research. DG, ÖMT and BÖ analyzed the data and performed the literature search. YK and BŞ provided oversight and were responsible for the study organization and implementation, and writing of the manuscript. BÖ and İY revised the manuscript’s intellectual content. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
All Patients with a Prognostic Role of Lymphadenectomy in Women with Low-Grade Lymphovascular Space Invasion-Positive Clinically Limited Endometrioid Endometrial Cancer signed an informed consent form before the procedure and was approved by the ethics committee of Ondokuz Mayıs University Hospital Medical Ethics Committee (Approval number: 10). The sample data analyzed for this manuscript were completely retrospective, with no patient or patient-related identifier included in the analysis.
We thank the staff in the Ondokuz Mayıs university for their assistance in conducting this clinical research study.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
