- Academic Editor
Background: This study aimed to evaluate the effectiveness of pelvic
and para-aortic lymphadenectomy in patients with advanced epithelial ovarian
cancer following neoadjuvant chemotherapy. Methods: This single-center
retrospective study analyzed patients with advanced epithelial ovarian cancer who
underwent interval debulking surgery between December 2008 and March 2020.
Patients’ clinical and pathological data were obtained from medical records.
Statistical analyses were performed using the Fisher’s exact and Mann–Whitney U
test. Results: Overall, 33 and 22 patients were included in the
lymphadenectomy and no-lymphadenectomy groups, respectively. The lymphadenectomy
group had longer operative time and higher rates of intraoperative blood loss and
lymph cysts than the no-lymphadenectomy group (p
The number of patients with ovarian cancer has been increasing globally. In 2021, approximately 21,410 women were diagnosed with ovarian cancer, whereas 13,770 died of this disease [1]. The 5-year relative survival rate of ovarian cancer is 49.1%, making it the most common cause of death among gynecologic malignancies. Generally, the two procedures for managing advanced ovarian cancer are primary debulking surgery followed by chemotherapy and neoadjuvant chemotherapy (NACT) followed by interval debulking surgery (IDS). Debulking surgery is an important procedure in the treatment of advanced ovarian cancer, as maximal cytoreduction should be the objective of primary surgery [2]. Systematic pelvic and para-aortic lymphadenectomy, as part of primary debulking surgery, has been previously reported to significantly improve survival in patients with advanced ovarian cancer [3]. The Lymphadenectomy in Ovarian Neoplasms (LION) trial, a prospective randomized the Arbeitsgemeinschaft Gynäkologisch Onkologie (AGO) study led by the Gynecologic Cancer InterGroup, is a multicenter randomized controlled trial comparing systematic pelvic and para-aortic lymphadenectomy with no-lymphadenectomy in patients with advanced ovarian cancer and normal lymph nodes. This trial showed that surgery with systematic pelvic and para-aortic lymphadenectomy was not associated with more prolonged overall survival (OS) or progression-free survival (PFS) than surgery without lymphadenectomy [4]. Lymphadenectomy was recommended for patients with advanced ovarian cancer who had enlarged lymph nodes and had undergone complete surgery [5]. Considering the previously reported usefulness of systematic lymphadenectomy, we performed systematic pelvic and para-aortic lymphadenectomy in patients with advanced ovarian cancer who had undergone macroscopically complete resection, regardless of lymph node enlargement after NACT.
Whether lymphadenectomy should be performed before NACT for advanced epithelial ovarian cancer with enlarged lymph nodes remains to be clarified despite the reduction in the size of lymph nodes. Notably, the significance of systematic pelvic and para-aortic lymphadenectomy in patients with advanced ovarian cancer is poorly documented in Japan. Therefore, this study aimed to evaluate the effectiveness of pelvic and para-aortic lymphadenectomy in patients with advanced epithelial ovarian cancer following NACT.
Patients with advanced epithelial ovarian cancer (International Federation of
Gynecology and Obstetrics [FIGO] stages IIB–IV) were included in this
single-center retrospective study. The patients underwent IDS following NACT
between January 2008 and March 2020 at the Mie University Hospital. The 2014 FIGO
classification was used to classify the patients. Using the predictive index,
patients with advanced ovarian cancer who were expected to be unfit to undergo
complete resection were treated with NACT and IDS [6]. NACT involved six cycles
of paclitaxel (175 mg/m
Additionally, patients who underwent IDS with systematic pelvic and para-aortic lymphadenectomy (lymphadenectomy [LNE] group) and those who underwent IDS without lymphadenectomy (no-lymphadenectomy [no-LNE] group) were identified. In the LNE group, pelvic and para-aortic lymphadenectomy was performed to the level of the left renal vein. Systematic lymphadenectomy was not performed in patients with poor PS or who had not undergone complete resection of residual tumor, and those who underwent suboptimal resection were excluded. All patients were administered with enoxaparin subcutaneously at 2000 IU every 12 hours, starting from 24 hours postoperatively until discharge.
Clinical and pathological data were obtained from patients’ medical records.
Clinical data included age, body mass index, surgical procedures, perioperative
and postoperative complications, operative time, blood loss, postoperative stay,
lymph node count, lymph node status, resection status, primary systematic
chemotherapy, and prognostic information. Lymph nodes with a short diameter
Statistical analysis was performed using the Fisher’s exact and Mann–Whitney U
test. Survival was assessed using the Kaplan–Meier method and compared between
the two groups using the log-rank test. The hazard ratio (HR) and associated 95%
confidence interval (CI) were calculated using a stratified Cox proportional
hazards model to identify PFS and OS, independent predictors. Factors inputted in
the univariate and multivariate analyses included age, stage, histology, presence
or absence of lymphadenectomy, residual tumor following IDS, and histology.
Statistical significance was set at p
Overall, the LNE and no-LNE groups comprised 33 and 22 patients, respectively. Clinical and pathological data are presented in Table 1. Fisher’s exact test revealed no significant differences between the two groups analyzed.
| LNE group (n = 33) | No-LNE group (n = 22) | p-value | ||
|---|---|---|---|---|
| Age (years) | 56.0 (48.0–65.0) | 59.0 (53.5–69.0) | 0.050 | |
| Body mass index | 21.4 (17.2–23.9) | 19.9 (17.8–22.5) | 0.533 | |
| Histological type | ||||
| Serous carcinoma | 25 (75.7) | 20 (90.9) | 0.284 | |
| Endometrioid carcinoma | 2 (6.0) | 1 (4.5) | ||
| Mucinous carcinoma | 2 (6.0) | 0 | ||
| Low-grade serous carcinoma | 3 (9.0) | 0 | ||
| FIGO stage | ||||
| IIB | 12 (6.0) | 1 (4.5) | ||
| IIIB | 3 (9.0) | 1 (4.5) | ||
| IIIC | 19 (57.5) | 13 (59.1) | ||
| IVA | 1 (3.0) | 1 (4.5) | ||
| IVB | 1 (3.0) | 2 (9.0) | ||
| Neoadjuvant chemotherapy cycles | 6 (6–6) | 6 (6–6.2) | 0.682 | |
| Enlarged lymph nodes | 12 (36.3) | 6 (27.2) | 0.565 | |
| Bevacizumab administration | 12 (36.3) | 9 (40.9) | 0.476 | |
| PARP inhibitor administration | 5 (15.1) | 4 (18.1) | 0.522 | |
| CA125 (U/mL) | 9.2 (6.5–15.9) | 13.0 (7.7–22.6) | 0.783 | |
Values are presented as median (range) or n (%).
CA125, cancer antigen 125; LNE, systematic lymphadenectomy; FIGO, International Federation of Gynecology and Obstetrics; PARP, poly(adenosine diphosphate)–ribose polymerase.
Table 2 shows the surgical findings and outcomes; however, no significant
differences were observed in surgical procedures between the two groups. In the
LNE group, the median number of pelvic and para-aortic lymph nodes resected was
33 (range, 23–33), and 33 (range, 16–31), respectively. Systematic
lymphadenectomy was performed appropriately. Complete resection was more likely
to be performed in the LNE group than in the no-LNE group. The median operative
time and amount of blood loss in both groups were significantly different
(p
| LNE group (n = 33) | No-LNE group (n = 22) | p-value | ||
|---|---|---|---|---|
| Surgery | ||||
| Bilateral salpingo-oophorectomy | 33 (100) | 22 (100) | ||
| Total hysterectomy | 33 (100) | 20 (90.9) | 0.156 | |
| Omentectomy | 33 (100) | 21 (95.4) | 0.400 | |
| Intestinal tract resection | 6 (18.1) | 1 (4.5) | 0.222 | |
| Splenectomy | 1 (3.0) | 0 | ||
| Hepatectomy | 1 (3.0) | 0 | ||
| Other | 1 (3.0) | 0 | ||
| Median of pelvic lymph nodes | 33 (23–33) | |||
| Median of para-aortic lymph nodes | 33 (16–31) | |||
| Complete resection performed | 28 (84.8) | 11 (50.0) | 0.007 | |
| Optimal resection performed | 5 (15.1) | 11 (50.0) | ||
| Operating time (min) | 370 (324–440) | 242 (190–292) | ||
| Blood loss (mL) | 763 (660–1204) | 431 (292–532) | 0.002 | |
| Blood transfusion | 21 (63.6) | 6 (27.2) | 0.008 | |
| Fever with body temperature |
13 (39.3) | 9 (40.9) | 0.565 | |
| Infection treated with antibiotics | 4 (12.1) | 0 | 0.120 | |
| Bowel obstruction | 5 (15.1) | 1 (4.5) | 0.218 | |
| Intensive care unit admission | 4 (12.1) | 3 (13.6) | 0.589 | |
| Thrombosis | 2 (6.0) | 0 | 0.356 | |
| Peripheral sensory neurologic event | 3 (9.0) | 0 | 0.208 | |
| Peripheral motor neurologic event | 2 (6.9) | 0 | 0.356 | |
| Asymptomatic lymph cysts | 25 (75.7) | 0 | ||
| Symptomatic lymph cysts | 6 (18.1) | 0 | 0.038 | |
Values are presented as median (range) or n (%).
LNE, systematic lymphadenectomy.
The median OS was 76 and 65 months in the LNE and no-LNE groups, respectively (HR = 1.31, 95% CI = 0.621–2.789, log-rank p = 0.470). The median PFS was significantly longer in the LNE group than in the no-LNE group (37.0 vs. 20.0 months, HR = 1.91, 95% CI = 1.037–3.531, log-rank p = 0.038). The Kaplan–Meier curves for the OS and PFS of all patients are shown in Fig. 1A,B. In patients with enlarged lymph nodes before chemotherapy, the median OS was 79 and 28 months in the LNE and no-LNE groups, respectively (HR = 5.361, 95% CI = 0.864–33.270, log-rank p = 0.071). The median PFS was considerably longer in the LNE group than in the no-LNE group (36.0 vs. 15.0 months, HR = 5.272, 95% CI = 1.410–19.714, log-rank p = 0.013). The Kaplan–Meier curves for the OS and PFS of patients with enlarged lymph nodes before chemotherapy are shown in Fig. 2A,B.
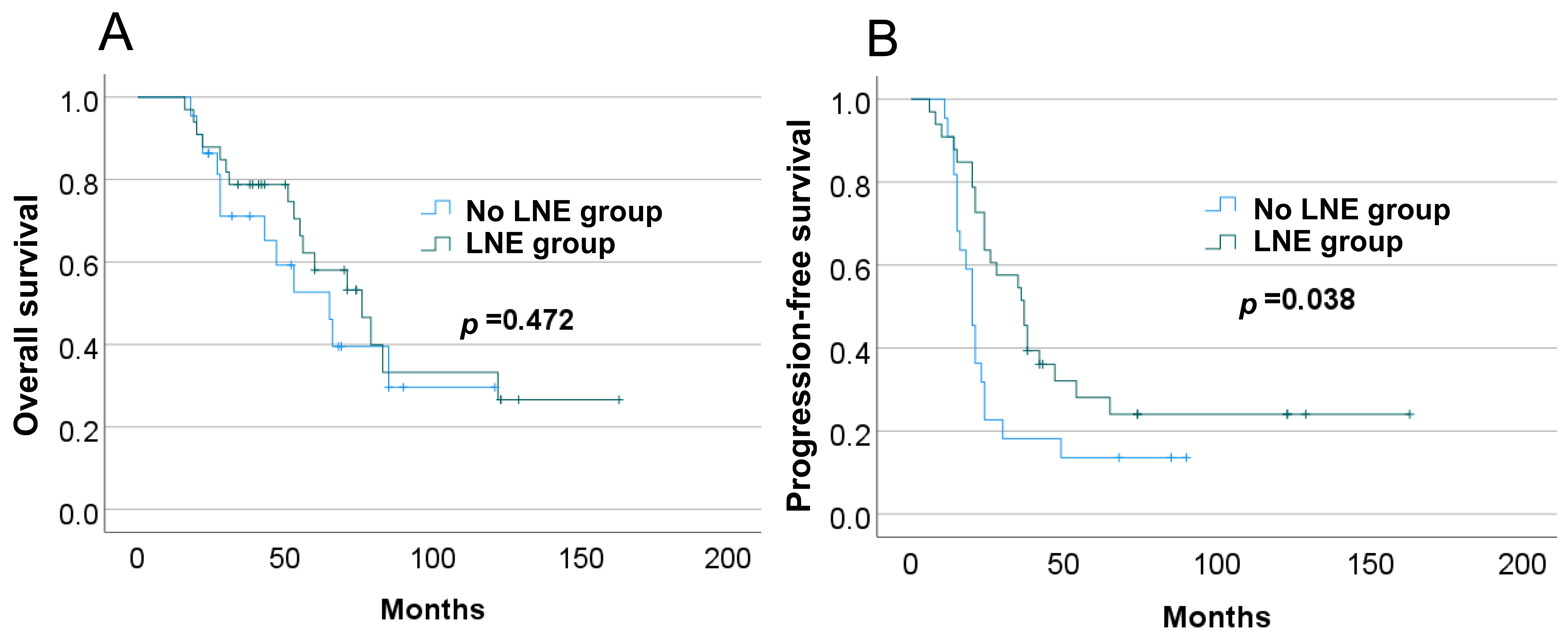 Fig. 1.
Fig. 1.Survival curves for the sensitivity analysis of all patients. Kaplan–Meier plots for the overall survival (A) and progression-free survival (B) of the LNE and no-LNE group. LNE, systematic lymphadenectomy.
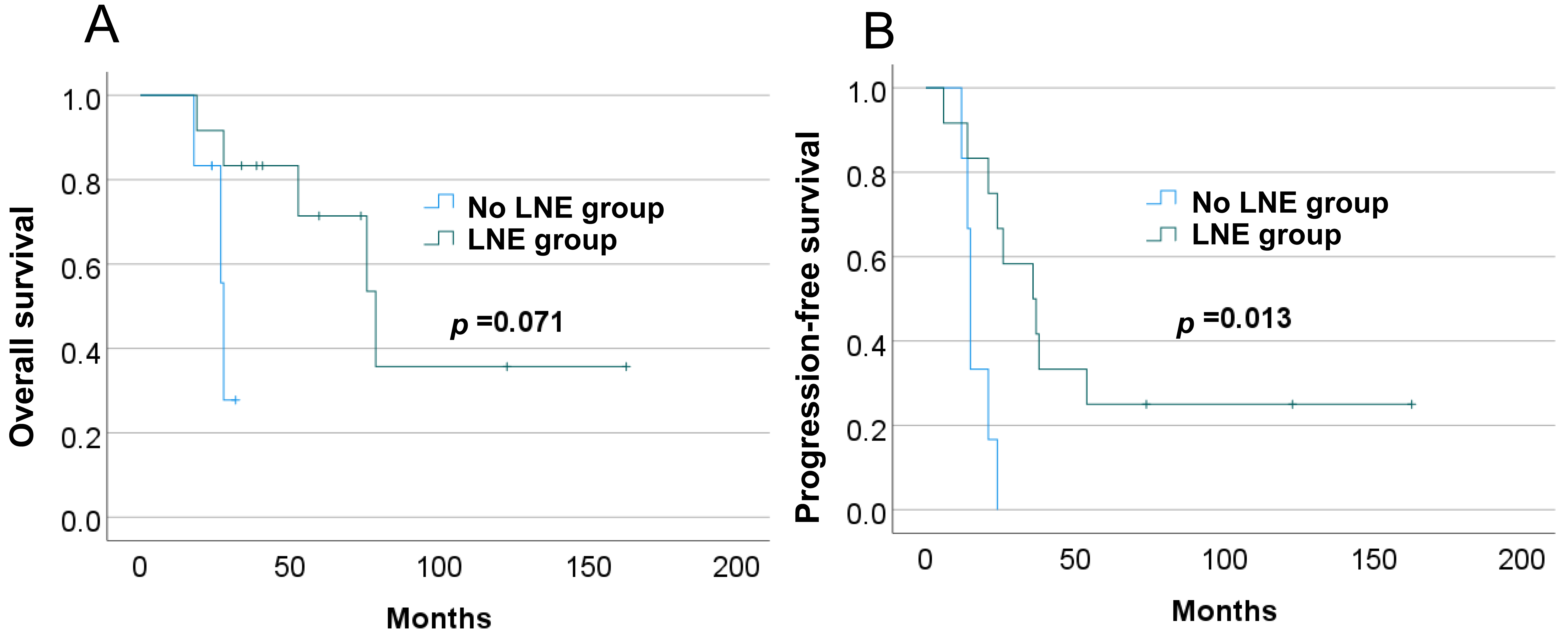 Fig. 2.
Fig. 2.Survival curves for the sensitivity analysis of patients with enlarged lymph nodes before chemotherapy. Kaplan–Meier plots for the overall survival (A) and progression-free survival (B) of the LNE and no-LNE groups. LNE, systematic lymphadenectomy.
Univariate analysis of all patients showed that residual tumor status (R0 vs. R1/2, HR = 2.379, 95% CI = 1.228–4.610, p = 0.010) and histology (serous vs. others, HR = 1.903, 95% CI = 0.981–3.691, log-rank p = 0.057) were associated with PFS (Table 3). Moreover, multivariate analysis of all patients revealed that residual tumor status and histology were related to PFS. Univariate and multivariate analyses of patients with enlarged lymph nodes before chemotherapy showed that lymphadenectomy (yes vs. no, HR = 0.161, 95% CI = 0.036–0.721, log-rank p = 0.017) and histology (serous vs. others, HR = 4.751, 95% CI = 1.263–17.879, log-rank p = 0.021) were associated with PFS (Table 4).
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age ( |
1.486 | 0.730–3.027 | 0.275 | - | - | - |
| Stage (II/III, n = 39; IV, n = 16) | 2.084 | 1.101–3.946 | 0.024 | - | - | - |
| Lymphadenectomy (yes, n = 33; no, n = 22) | 0.522 | 0.283–0.964 | 0.038 | - | - | - |
| Residual tumor status (R0, n = 39; R1/2, n = 16) | 2.379 | 1.228–4.610 | 0.010 | 3.142 | 1.539–6.419 | 0.022 |
| Enlarged lymph nodes (yes, n = 18; no, n = 37) | 1.208 | 0.645–2.263 | 0.555 | - | - | - |
| Histology (serous, n = 40; others, n = 15) | 1.903 | 0.981–3.691 | 0.057 | 2.590 | 1.267–5.294 | 0.009 |
| Bevacizumab administration (yes, n = 21; no, n = 34) | 0.956 | 0.516–1.773 | 0.087 | - | - | - |
| PARP inhibitor administration (yes, n = 9; no, n = 46) | 0.523 | 0.247–1.110 | 0.091 | - | - | - |
HR, hazard ratio; CI, confidence interval; PARP, poly(adenosine diphosphate)–ribose polymerase.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age ( |
1.152 | 0.323–4.102 | 0.827 | - | - | - |
| Stage (II/III, n = 10; IV, n = 8) | 1.362 | 0.490–3.782 | 0.554 | - | - | - |
| Lymphadenectomy (yes, n = 12; no, n = 6) | 0.190 | 0.051–0.709 | 0.013 | 0.161 | 0.036–0.721 | 0.017 |
| Residual tumor status (R0, n = 14; R1/2, n = 4) | 2.356 | 0.717–7.734 | 0.158 | - | - | - |
| Histology (serous, n = 10; others, n = 8) | 4.303 | 1.256–13.741 | 0.020 | 4.751 | 1.263–17.879 | 0.021 |
| Bevacizumab administration (yes, n = 5; no, n = 13) | 0.709 | 0.230–2.185 | 0.559 | - | - | - |
| PARP inhibitor administration (yes, n = 2; no, n = 16) | 0.093 | 0.013–0.677 | 0.019 | - | - | - |
HR, hazard ratio; CI, confidence interval; PARP, poly(adenosine diphosphate)–ribose polymerase.
This study analyzed the effectiveness of pelvic and para-aortic lymphadenectomy in patients with advanced epithelial ovarian cancer following NACT, and results showed that systematic pelvic and para-aortic lymphadenectomy in patients with advanced epithelial ovarian cancer caused complications. In patients with enlarged lymph nodes before chemotherapy, systematic lymphadenectomy did not prolong OS; however, it significantly prolonged PFS. Multivariate analysis revealed that residual tumor status and histology were associated with PFS in all patients and that systematic lymphadenectomy and histology were associated with PFS in patients with enlarged lymph nodes before chemotherapy.
Many studies have been conducted on systematic lymphadenectomy in advanced ovarian cancer [4, 9, 10]. For example, Du Bois et al. [10] reported that in advanced ovarian cancer, the systematic lymphadenectomy group had prolonged OS in patients with complete resection, even in the absence of preoperative or intraoperative lymph node enlargement. However, the usefulness of systematic lymphadenectomy was difficult to assess because of the bias to omit lymphadenectomy based on age, PS, stage, and other patient factors. Therefore, in the LION trial, systematic lymphadenectomy did not prolong OS or PFS in patients with advanced ovarian cancer who had undergone complete macroscopic resection. Furthermore, it was associated with complications, such as longer operative time, greater blood loss, increased intensive care unit admissions, and higher rates of blood transfusions, infections, lymph cysts, and 60-day mortality [4]. Similar to the LION trial, in our study, systematic lymphadenectomy did not prolong the OS and resulted in more perioperative complications, such as increased operative time, blood loss, blood transfusions, and lymphatic cysts in the lymphadenectomy group; however, it prolonged the PFS.
The LION trial was conducted on patients with advanced ovarian cancer without enlarged lymph nodes before chemotherapy. Conversely, several studies reported on systematic lymphadenectomy in patients with advanced ovarian cancer and enlarged lymph nodes before chemotherapy. In addition, Panici et al. [11] reported that in advanced ovarian cancer with enlarged lymph nodes, the risk of recurrence was significantly lower in the systematic lymphadenectomy group than in the group where only resection of bulky nodes was performed. Therefore, the resection of clinically suspicious or bulky nodes should be indicated in all patients with advanced ovarian cancer if complete resection is possible [12, 13].
In contrast, no gross residual tumor has independently significant factors associated with the prognosis for advanced-stage ovarian cancer [14]. Recently, in phase 3 of the PAOLA-1 (PAOLA-1/ENGOT-ov25) trial [15], bevacizumab, a vascular endothelial growth factor A–targeting monoclonal antibody), poly(adenosine diphosphate)–ribose polymerase (PARP) inhibitors, and immune checkpoint inhibitors were administered to patients with ovarian cancer in combination with chemotherapy or as maintenance therapy. For patients in this trial who had undergone cytoreductive surgery without residual macroscopic tumor, PFS was significantly longer in the olaparib group than in the placebo group (HR for disease progression or death, 0.54; 95% CI = 0.42–0.71).
In our study, a good prognosis for PFS was associated with complete resection and histology of serous carcinoma in all patients, including systematic lymphadenectomy and histology of serous carcinoma in patients with enlarged lymph nodes. High-grade serous ovarian cancer has a high response rate to platinum-based chemotherapy; therefore, serous carcinoma was considered a good prognostic factor in patients with advanced ovarian cancer treated with preoperative chemotherapy [16, 17]. In patients with advanced ovarian cancer with enlarged lymph nodes and who had undergone complete resection surgery, systematic lymphadenectomy was considered to be associated with a good prognosis.
Several studies have investigated systematic lymphadenectomy with IDS after NACT to manage advanced ovarian cancer [18, 19, 20, 21]. For example, Fagotti et al. [18] reported that systematic pelvic lymphadenectomy and para-aortic lymphadenectomy during IDS had no value in improving PFS and OS; this procedure resulted in higher rates of complications, such as longer operative time, increased blood transfusions, and occurrence of lymphoceles. Furthermore, Song and Gao reported that the extent of lymphadenectomy (systematic or selective) had no significant impact on PFS or OS [19].
In this study, systematic lymphadenectomy prolonged PFS when IDS was performed after NACT in advanced ovarian cancer with enlarged lymph nodes before chemotherapy. In previous reports, the median number of NACT cycles varied from three to six courses. Because our report demonstrated the benefits of six NACT cycles for the high rate of complete resection or optimal IDS [22], our institution has been performing six preoperative and postoperative chemotherapy courses. Because of differences in treatment backgrounds, the studies’ results and prognoses may differ.
Recently, the addition of hyperthermic intraperitoneal chemotherapy to IDS has been reported to improve PFS and OS, have no high rate of complications, and is expected to be a treatment option in the future [23].
This study has some limitations. First, this was a retrospective study conducted at a single institution. Therefore, to reduce selection bias, prospective randomized trials investigating systematic pelvic and para-aortic lymphadenectomy in patients with advanced ovarian cancer should be conducted. Second, data from cases with different treatment strategies for ovarian cancer were used in our study. In addition, this study lacks data on germline BRCA mutation and homologous recombination deficiency status (HRD). We included data from 2008 cases treated with bevacizumab and a few cases treated with PARP inhibitors. The usefulness of PARP inhibitors for patients with HRD-positive tumors and germline BRCA mutation has been reported [15, 24]. Considering the results of this study, we would like to clarify germline BRCA mutation and HRD status and examine ovarian cancer cases treated with PARP inhibitors. The role of systematic lymphadenectomy should also be considered in the context of treatment strategies for ovarian cancer. However, prolonged PFS with appropriate systematic lymphadenectomy may prolong the platinum-free interval and contribute to treatment with PARP inhibitors.
We would conduct prospective randomized trials investigating systematic pelvic and para-aortic lymphadenectomy in patients with advanced ovarian cancer with enlarged lymph nodes and clarify the correlation of germline BRCA mutation and HRD status with clinical and survival outcomes in patients with advanced-stage ovarian cancer.
Patients who underwent lymphadenectomy had more perioperative complications than those who did not undergo this procedure. However, systematic lymphadenectomy significantly prolonged PFS, particularly in patients with enlarged lymph nodes. The significance of lymphadenectomy in advanced epithelial ovarian cancer with enlarged lymph nodes before NACT has not been elucidated; nonetheless, systematic lymphadenectomy after NACT has the potential to improve prognosis in especially patients with ovarian cancer who had undergone complete resection with enlarged lymph nodes.
The data that support the findings of this study are available from the corresponding author (EK) upon reasonable request.
MKK and EK designed the research study. MKK performed the research. MKK, EK, NE, KO, KT, MN, KY and TI analyzed the data. MKK and EK wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
All participants provided informed consent for inclusion before participating in this study. This study was conducted following the Declaration of Helsinki, and the Clinical Research Ethics Review Committee of the Mie University Hospital approved the protocol (approval number: H2022-038). Informed consent was obtained in the form of an opt-out on the website.
The authors would like to thank Editage (https://www.editage.com) for English language editing.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
