- Academic Editor
Background: Empty follicle syndrome (EFS) is a rare complication in
which no oocytes are retrieved in oocyte pick-up (OPU) despite adequate
controlled ovarian hyperstimulation (COH). Various studies and systematic reviews
have reported that EFS is mainly caused by diminished ovarian reserve (DOR)
because EFS tends to occur in patients with a poor response to COH. However,
these factors do not explain all cases. Current knowledge of these pathological
factors is limited, and treatment is unknown. This study aimed to find out the
clinical predictors of EFS before OPU. Methods: In this study, 2342
cycles of 1148 cases that underwent OPU between January 2015 and November 2020 in
two reproductive clinics were retrospectively enrolled. Ninety-one and 2251
cycles were classified as EFS (no cultivatable oocytes retrieved) and non-EFS
(cultivatable oocytes retrieved), respectively. Results: The EFS and
non-EFS incidence was 3.9% and 96.1%, respectively. The mean patient age in the
EFS group was higher than that in the non-EFS group (40.3
Empty follicle syndrome (EFS) is a condition in which oocytes cannot be
retrieved from mature follicles after ovulation induction [1]. Failure to
retrieve cultivatable oocytes is a major barrier to the smooth progress of
assisted reproductive technology (ART). Thus, many studies have been performed to
elucidate the pathogenesis of EFS; however, a definition is yet to be
established. EFS is sometimes classified as either genuine or false by estimating
the maturity of follicles by monitoring beta-human chorionic gonadotropin
(
The expected number of oocytes to be retrieved is an important indicator for ART. More precisely, medical providers and patients are concerned about whether the retrieved oocytes are cultivatable. However, it is possible to select only cases in which multiple oocytes are expected to be retrieved and reduce the total number of OPUs (highly invasive procedures) if the number of cultivatable oocytes can be predicted before retrieval. Therefore, we analyzed patients who underwent OPU to search for factors affecting the results and evaluated the incidence of EFS for each number of follicles just before OPU. Our objective was to detect the predictors for EFS with the multivariate analysis through this double-center retrospective cohort study of 2342 cycles of 1148 cases that underwent OPU. Furthermore, we simultaneously performed the analysis in the population in which cases of diminished ovarian reserve (DOR) were excluded based on recent reports on the possibility of the causal influence of DOR on EFS [6, 7].
A total of 1148 cases undergoing OPU, for which sufficient clinical information
was obtained, were analyzed at the Department of Reproductive Medicine of Kameda
General Hospital and Kameda IVF Clinic, Makuhari, Japan, from January 2015 to
November 2020. The results of oocyte retrieval were divided into two groups:
those in which cultivatable oocytes could not be retrieved (EFS) and those in
which they could be retrieved (non-EFS). This study excluded cases of premature
luteinization, defined as a rise in luteinizing hormone (LH) levels accompanied
by a progesterone rise
Controlled ovarian stimulation for OPU was mainly performed by fixed GnRH
antagonist protocol. From the second or third day of menstruation, stimulation
was initiated by administering a daily injection of 150–300 U of urinary human
menopausal gonadotropin (HMG, Ferring Pharmaceuticals Co., Ltd., Tokyo, Japan) or
recombinant FSH (Gonalef®, Merck Biopharma Co., Ltd., Tokyo,
Japan) in combination with 50 mg of clomifene citrate (Clomid®,
Fuji Pharma Co., Ltd., Tokyo, Japan) twice daily for 5 days. Cetrorelix acetate
(Cetrotide®, Nippon Kayaku Co., Ltd., Tokyo, Japan) or ganirelix
acetate (GANIREST®, Merck & Co., Inc., Kenilworth, NJ, USA) was
administered as a subcutaneous injection of 0.25 mg, a GnRH antagonist, either on
the fifth day of stimulation or when the leading follicle had reached a diameter
of 15 mm, whichever occurred earlier. The ovulation trigger was mainly
administered with 250
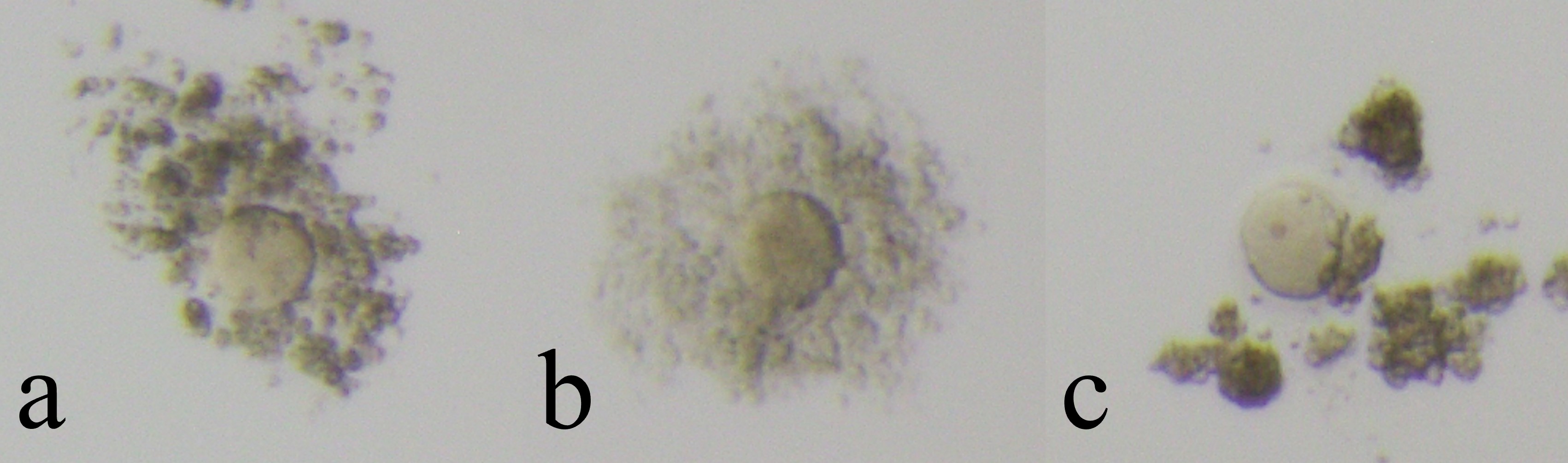 Fig. 1.
Fig. 1.Cultivatable oocytes (non-Empty Follicle Syndrome oocytes). Cultivatable oocytes have intact zona pellucida and viable cytoplasm. Oocytes were assessed by its coronal cells and divided into three groups (a: immature, b: mature, c: over mature).
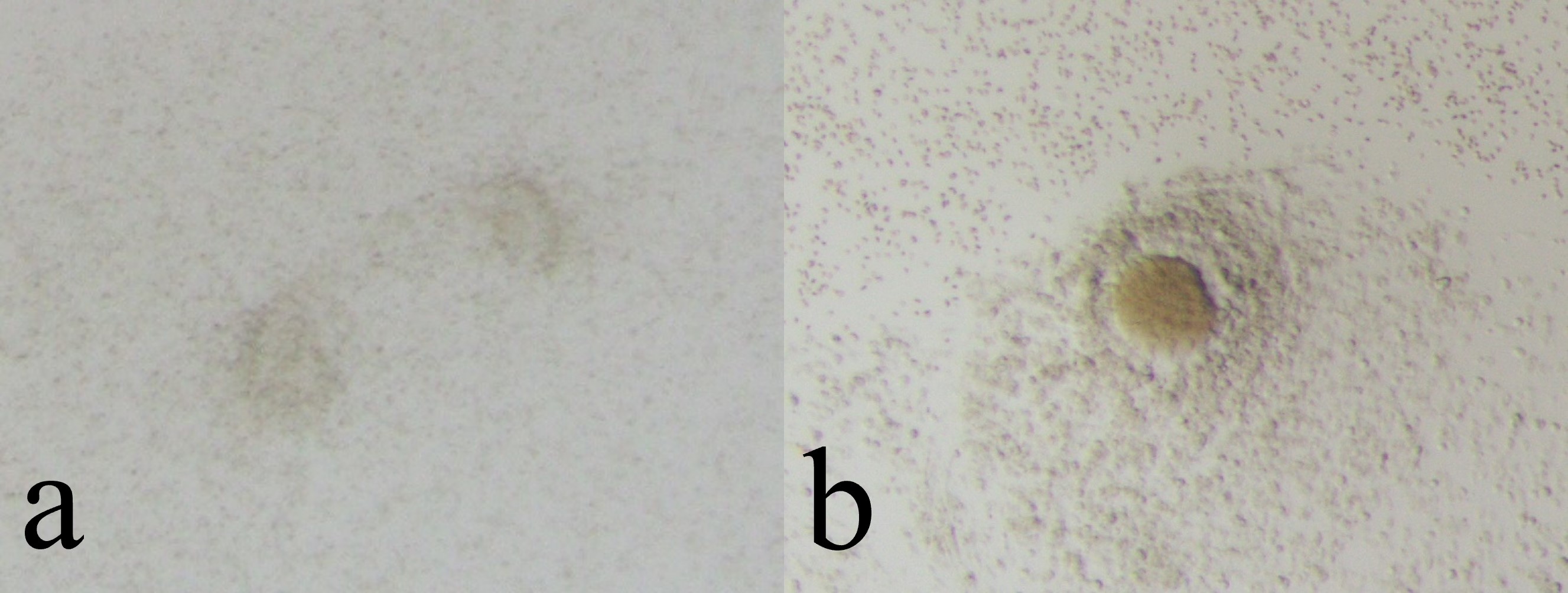 Fig. 2.
Fig. 2.Non-cultivatable oocytes (Empty Follicle Syndrome oocytes). Non-cultivatable oocytes have no cytoplasm (a) or only have degenerated cytoplasm (b).
The following parameters were analyzed in two groups by Welch’s T-test:
age at retrieval, body mass index (BMI), anti-Müllerian hormone (AMH) levels,
antral follicle count (AFC), and the number of follicles whose diameter is larger
than 13 mm on the day of oocyte retrievals. Statistical significance was set at
p
Additionally, analyses were conducted on a population excluding those with DOR,
which was defined according to POSEIDON criteria as having AFC
Table 1 shows the distribution of age at OPU, BMI, AMH levels, AFC, and the number of follicles for cases in the two groups.
| Total | EFS (n = 91) mean |
non-EFS (n = 2251) mean |
range (min–max) | p-value | |
|---|---|---|---|---|---|
| Age (years) | 38.0 |
40.3 |
37.9 |
23–48 | |
| BMI (kg/m |
22.0 |
21.7 |
22.0 |
14.4–37.3 | 0.52 |
| AMH (ng/mL) | 2.7 |
1.0 |
2.7 |
0.02–21.5 | |
| AFC | 8.3 |
2.9 |
8.5 |
0–100 | |
| Follicles (No.) | 5.8 |
1.9 |
6.0 |
1–45 |
EFS, empty follicle syndrome; AMH, anti-Müllerian hormone; AFC, antral follicle count; BMI, body mass index; SD, standard deviation.
There were 91 (3.9%) and 2251 (96.1%) EFS and non-EFS cases, respectively: age
at retrieval was 40.3
Odds ratios for the effect of each parameter on non-EFS are shown with 95% confidence intervals (Fig. 3). The odds ratios for AFC and number of follicles were significant for non-EFS cases (1.301 and 1.832, respectively), indicating that higher AFC or number of follicles mean a higher likelihood of retrieving cultivatable oocytes. The odds ratios for age, BMI, and AMH levels were not significant. On comparing the EFS and non-EFS groups, the cases in the EFS group were older and had lower AMH levels, AFC, and number of follicles. when the percentage of women with EFS was calculated for each number of follicles, there was a decreasing trend: 21.2%, 7.8%, 2.7%, and 1.2% for 1, 2, 3, and 4 follicles, respectively (Fig. 4). Thereafter, the incidence leveled off, but none of the 410 cases with 10 or more oocysts had EFS. In other words, ensuring a high number of follicles, regardless of age, may reduce the likelihood of EFS.
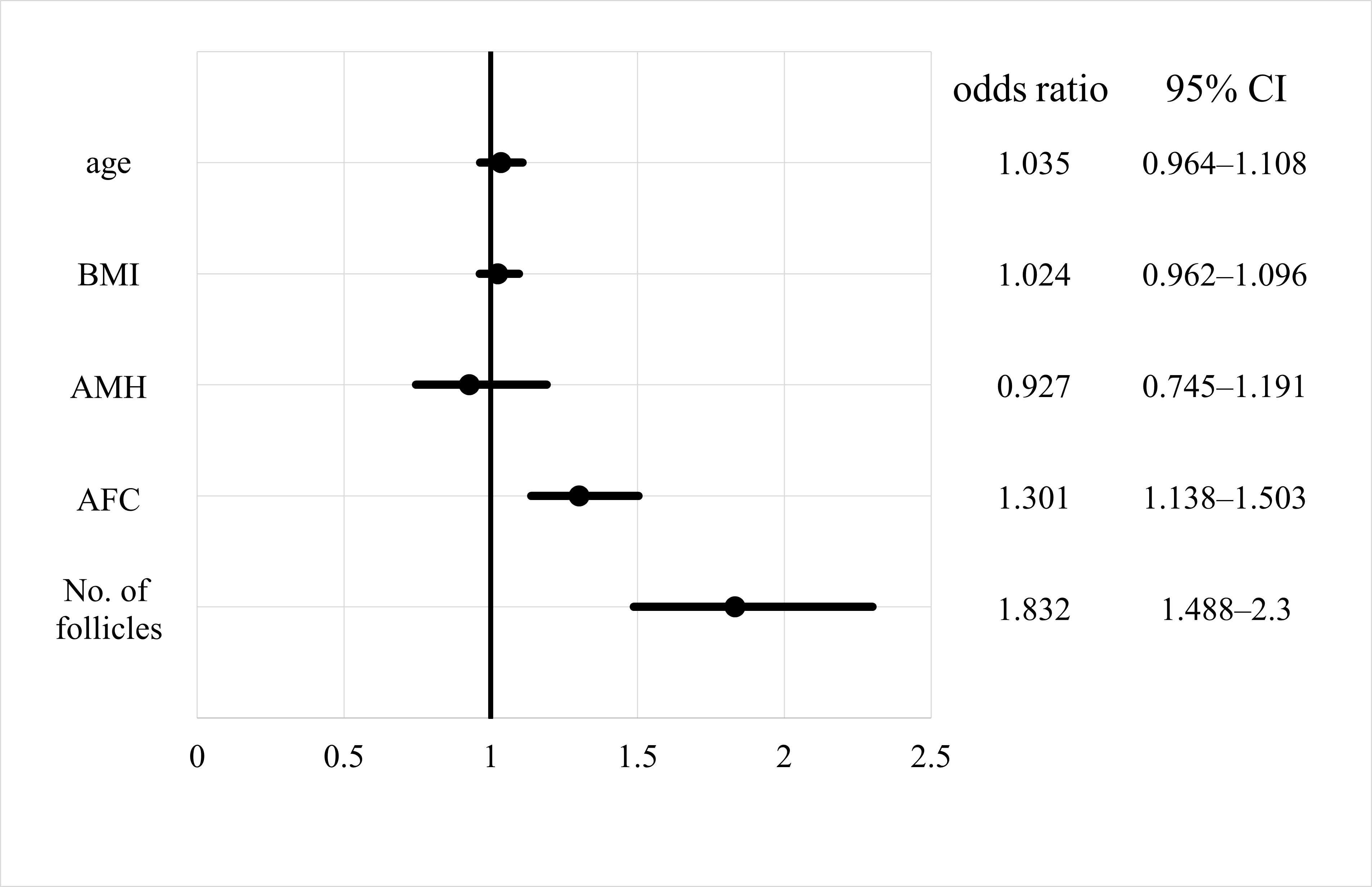 Fig. 3.
Fig. 3.Odds ratio of empty follicle syndrome for each factor. CI, confidence interval; AMH, anti-Müllerian hormone; AFC, antral follicle count; BMI, body mass index.
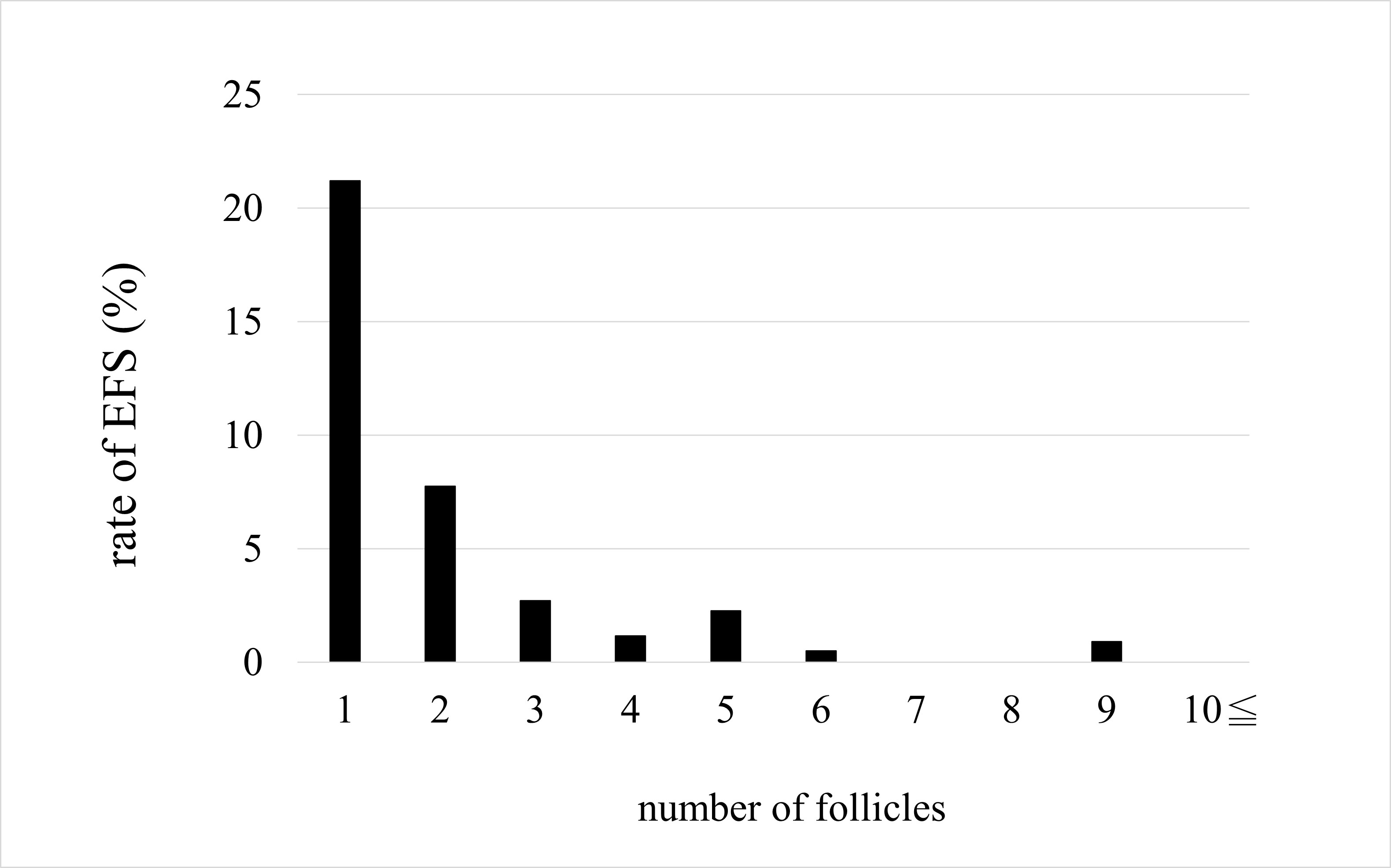 Fig. 4.
Fig. 4.Rate of empty follicle syndrome (EFS).
Both the AFC and number of follicles, when DOR was excluded, showed significant
differences between the two groups (p
 Fig. 5.
Fig. 5.Odds ratio of empty follicle syndrome for each factor when cases of diminished ovarian reserve are excluded. CI, confidence interval; AMH, anti-Müllerian hormone; AFC, antral follicle count; BMI, body mass index.
This study showed that the likelihood of retrieving cultivatable oocytes increases with increasing AFC and the number of follicles at OPU. However, AMH was not a predictor of the number of cultivatable oocytes.
EFS has been debated in multiple respects since it was first reported in 1986 [1]. EFS is defined as failure to retrieve oocytes from mature follicles after ovulation induction by puncture, aspiration, and flushing. It is not clear how the pathology works when no oocytes are retrieved after hCG administration. In sisters with congenital deafness who developed genuine EFS [9], whole genome sequencing of the family lineage revealed that missense mutations in the luteinizing hormone choriogonadotropin receptor led to the inhibition of follicle development [10]. Furthermore, a case of EFS with a different homozygous mutation of the same receptor [11] or with a pericentric inversion on chromosome 2, 46, XX, inv (2) (p11q21) [12] was also reported. However, these cases are rare, and several reports have stated that the main cause is ovarian aging, in other words, patient age at OPU [2, 3, 4, 5].
In this study, comparing the EFS and non-EFS groups, the patients in the EFS group were older and had lower AMH levels, AFC, and number of follicles, although ensuring a high number of follicles, regardless of age, may reduce the likelihood of EFS. Thus, the higher AFC and number of follicles measuring greater than 13 mm on the day of OPU indicated an increased probability of retrieving cultivatable oocytes. Therefore, even if the AFC are low at the beginning of the menstrual cycle, the probability of retrieving cultivatable oocytes may be raised by increasing the number of follicles through ovarian stimulation.
To the best of our knowledge, the evidence on EFS is fully unestablished, here we reviewed and discussed the literature as follows.
In a previous study, logistic regression analysis showed that AFC is an important factor in EFS [2], which is consistent with our findings. However, excluding patients with endometriosis, polycystic ovary syndrome, or previous ovarian surgery, the study was limited to 95 patients with 6 or fewer follicles measuring 14 mm or larger on the day of hCG administration. Moreover, the incidence of EFS (4.2%) was determined among patients with low ovarian response. Of these patients, only 4 were determined to have genuine EFS (age, 40–44 years). With logistic regression analysis of 56 cases that met the Bologna criteria post hoc, AFC was determined to be a major risk factor for EFS. Nevertheless, the paper did not discuss why age, which mainly influences ovarian aging, is not statistically significant. The authors even note that while the EFS criteria are more rigorous than those used in the present study and theirs had the advantage of being a prospective study, the small number of cases limits the generalization of the results.
Aktas et al. [3] analyzed 3060 oocyte retrievals in 1849 patients aged
21–44 years. As in the present study, the analysis was performed without
distinguishing between genuine and false EFS. The 25 cases (0.8%) in which no
oocytes were retrieved were designated as EFS, with the EFS group having a
significantly higher age (35 vs. 33 years, p
A previous study examined the recurrence rate of EFS, not by comparing the EFS
and non-EFS groups, but by dividing 35 cases with at least one occurrence of EFS
into three groups based on age:
Baum et al. [5] compared 163 EFS cases (those with follicles
Thus, the main cause of EFS has been considered to be older age, which almost equals DOR. However, the studies considering age as a risk factor have used mainly univariate analyses, with surprisingly few studies considering the confounding of each factor, and no consideration has been given to the lack of a significant difference in age in subclass analyses. The strength of the present study is the focus on this neglected contradiction.
On the other hand, Castillo et al. [13] compared the incidence of EFS
(defined as no oocytes retrieved at all) in 2034 cycles of oocyte donations and
1433 cycles of in vitro fertilization (IVF) patients. There was a
significant difference in the age distribution of the two groups (25 vs.
31 years, p = 0.01). These could be considered groups of young good
responders to ovarian stimulation and general IVF patients regardless of
response, respectively, with a comparable incidence of EFS (3.5% vs.
3.1%). However, this study was limited to IVF patients aged
A systematic review by Stevenson et al. [14] examined 34 case reports and studies from 1986 to 2006, excluding cases with no hCG data and others. The review concluded that age had no effect on EFS since the average age of patients with genuine EFS was 33 years and most had ovarian reserves within the normal range. Furthermore, the review pointed out that many of the case reports may have been due to unexpected early ovulation.
Taken together, previous studies were controversial results between EFS and age, although our study did not show the association between EFS and age with the multivariate analysis. Further study is needed in the larger-multi center randomized controlled protocol to elucidate whether the factor of age is associated with EFS.
There are a few reports on the association between EFS and AMH, in which AFC was more indicative of ovarian response than AMH [15, 16], although Younis stated that EFS should be examined using AMH as an indicator of ovarian reserve [17]. In our study, univariate analysis showed a significant difference in AMH between the two groups, as well as in age. However, multivariate analysis showed no significant differences. This does not support the idea that AMH is a risk factor for EFS because it represents DOR. Conversely, we performed multivariate analysis for a subpopulation in which cases of DOR were excluded from the main population of this study to clarify that DOR was not the cause of EFS. We found that the AFC and number of follicles were predictors with and without DOR, although age was added as a predictor. Thus, our analyses led us to conclude that it is unlikely that DOR is a cause of EFS.
This report has two strength points. For a long time now, risk factors for EFS
have been examined. Some studies used multivariate analyses but had small sample
sizes, and others had much larger sample sizes but only used
EFS is an event whose pathogenesis is still unknown. It is an obstacle to treatment for patients and healthcare providers. Moreover, it is especially stressful for patients psychologically, physically, and financially. EFS is defined as the inability to retrieve any oocyte at all, but in this study, we defined it as the inability to retrieve cultivatable oocytes. This is because, in the end, what is important in clinical practice is not whether oocytes can be recovered, but whether the recovered oocytes can be cultured. In clinical practice, indicating a relationship between the AFC or the number of follicles and the number of cultivatable oocytes retrieved is very useful for obtaining informed consent. As previously mentioned, several studies have examined the incidence of EFS in given populations; however, no study has focused on the percentage of cases in which clinically meaningful oocytes are not retrieved. This is the second strength of the current study.
There were several limitations in this study. First, EFS is classified as genuine or false EFS. Genuine EFS is defined as the inability to retrieve oocytes from punctured follicles despite the presence of sufficient hCG and mature follicles on the day of OPU. False EFS, on the other hand, is defined as the inability to retrieve oocytes under conditions of low hCG on the day of OPU and inadequate follicle development. However, the range of adequate hCG varies with each report and hence, the definition is ambiguous [18]. False EFS is caused by improper self-injection of fertility drugs due to inadequate explanation regarding the handling of injectable drugs by the medical provider or poor understanding or inexperience of the patient, resulting in an incorrect time of injection, or inadequate amount of hCG administration, unskilled oocyte extraction techniques, inadequate aspiration pressure, and malfunction of equipment [19]. In the present study, we did not distinguish between genuine and false EFS, and unfortunately, the limitation is that false EFS cannot be excluded although all procedures were almost performed according to established protocols under the supervision of certified physicians. This study is unique when compared with other studies as we analyzed not only cases in which no oocytes were retrieved at all, but also cases in which oocytes were retrieved but not cultivatable, termed as ‘clinical EFS’. Previous studies have only classified cases based on the number of oocytes retrieved, and no studies have examined retrieved oocytes qualitatively. However, what patients want is not the retrieval of just any oocytes, but more realistically, the retrieval of cultivatable oocytes. Second, flushing was performed with a single-lumen needle when the number of follicles was less than four in this study. In contrast, previous systematic reviews and meta-analyses failed to identify any clinical benefit of follicular flushing in woman undergoing IVF with normal [20] or poor [21] ovarian response. Additionally, a recent meta-analysis also concluded the uncertainty regarding the impact of follicular flushing in poor-responders on the number of retrieved oocytes, as two studies have supported a decrease in the number of oocytes, one study supported an increase, and another study demonstrated no significant differences with flushing [22]. Therefore, we sub-analyzed to determine whether follicular flushing increased or decreased the number of follicles. Interestingly, there was a significant difference between follicular flushing and non-flushing groups (Supplementary Fig. 1). However, a limitation of this study is that we did not distinguish between flushing and non-flushing groups. Third, a recent prospective study recommended that, in mono-follicular responder patients with DOR, the employment of a double-lumen needle for follicular flushing results in a greater yield of oocytes compared to aspiration and flushing of follicles with a single-lumen needle [23]. In this study, we used only a single-lumen needle to retrieve oocytes, and we considered that a single-lumen needle is one of the confounding factors as a limitation. Fourth, the aspiration pressure for oocyte retrieval is recommended to be below 120 mmHg [24], although one study reported that an aspiration pressure of 150 mmHg is safe for oocytes [25]. However, aspiration pressure was 270–290 mmHg in this study. Therefore, the possibility that the high level of aspiration pressure was the causal factor for EFS was considered, as it was harmful to the oocytes and decreased the number of oocytes retrieved.
Physicians as well as patients fear this complication associated with OPU, and knowledge of its rate in advance would be extremely important from the perspective of risk assessment and informed consent. EFS should be avoided as much as possible in ART. In choosing an ovarian stimulation method, the AFC or number of follicles at OPU should be considered more than age.
AFC, antral follicle count; AMH, anti-Müllerian hormone; ART, assisted reproductive technology; BMI, body mass index; COH, controlled ovarian hyperstimulation; DOR, diminished ovarian reserve; EFS, empty follicle syndrome; GnRH, gonadotropin-releasing hormone; hCG, human chorionic gonadotropin; IVF, in vitro fertilization; LH, luteinizing hormone; OPU, oocyte pick-up; SD, standard deviation.
The datasets analyzed during the current study are not publicly available due to a contract with the corresponding author but are available from the corresponding author on reasonable request.
JM, KOta, TI and KK designed the research study. JM, KH, SK, YT, YN, MT, KOhu and MH performed the research and provided help and advice on this research. JM, TS and HY analyzed the data. JM and TI wrote the manuscript. KOta revised the manuscript. KOta took responsibility for all aspects of the reliability of the data presented. All authors made critical revisions and gave final approval of the version to be published.
This study was approved by the Institutional Review Board of Kameda Medical Center and Kameda IVF Clinic Makuhari (17-018). The study follows the principles of the Declaration of Helsinki. Informed consent was obtained from all subjects involved in the study.
We gratefully acknowledge all the staff who worked during the pandemic. And we want to acknowledge a secretary Megumi Ishikawa for her encouragement during the writing of this manuscript.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
