- Academic Editor
†These authors contributed equally.
Background: To explore the effect of granulocyte colony-stimulating
factor (G-CSF) on thin endometrium in women undergoing assisted reproduction.
Methods: We performed a methodical search from their inception to
December 2022 in various electronic databases containing PubMed, Cochrane
Library, Embase, Web of Science, Scopus, in addition to a manual search. All
journals concerning the effect of G-CSF on thin endometrium were found. Selected
studies, collected data, and assessed risk of bias were conducted by two
investigators under precise inclusion and exclusion criteria independently. We
applied Revman 5.3 software to accomplish the Meta-analysis of qualified studies.
Results: This research included 8 studies, including 6 randomized
controlled trials and 2 non-randomized controlled studies, a total of 673
patients. Based on the meta-analysis, we noted that compared with the control
group, G-CSF significant improved embryo implantation rate [risk
ratio (RR) =1.91, 95% confidence interval (CI) (1.26, 2.91), p = 0.002]
and clinical pregnancy rate [RR = 1.73, 95% CI (1.22, 2.45),
p = 0.002]. Compared with the control group, the endometrial thickness
in the G-CSF group had non-significant increase compared with that of the control
group [mean difference (MD) = 0.81, 95% CI (–0.04, 1.67), p = 0.06],
in randomized controlled trial (RCT) studies, subgroup analysis shows G-CSF group
increased significantly [MD = 1.13, 95% CI (0.56, 1.67), p
Infertility is assessed to affect approximately 8–12% of husband and wife of
childbearing age worldwide [1]. Assisted reproductive technology (ART) has been
widely used in the treatment of infertility over the past few decades. In 2014,
about 1.93 million ART cycles were carried out in 76 countries around the world,
frozen embryo transfer cycles increased by about 2/3 compared with 2010 [2]. High
quality embryos and endometrial receptivity are both critical for a successful
pregnancy; however, endometrial receptivity may represent a key rate-limiting
factor for pregnancy rates in ART. An important marker of endometrial receptivity
is endometrial thickness; a thin endometrium is closely associated with a poor
pregnancy outcome after embryo transfer [3, 4, 5]. In ART, a thin endometrium is
often defined as an endometrial thickness
Clinically, there are several different approaches that considered to be helpful to treat patients with a thin endometrium, including aspirin, human chorionic gonadotropin, sildenafil citrate, estrogen, antioxidant, vitamin E, alpha-tocopherol, pentoxifylline, nifedipine and so on [8]. These treatments are selected based on the type of embryo transfer (ET) being planned. Nonetheless, embryo transfer cycles must be cancelled repeatedly due to some patients’ endometrium is still unresponsive after using these therapies, even embryo transferred may fail in implantation [8].
Granulocyte colony stimulating factor (G-CSF) is a cytokine that belongs to a large family of cytokines [9]. Previous studies have shown that G-CSF act vital function in endometrial growth and pregnancy. G-CSF may be involved in embryo implantation by regulating decidual macrophages and Th2 reaction [10]; G-CSF can induce the proliferation of endometrial epithelium and stromal cells by increasing the staining level of proliferating cell nuclear antigen, and promote the regeneration of thin endometrium [11]; G-CSF shown to stimulate the directed differentiation of stem cells at the site of injury and increase the thickness of the endometrium in rat models [11]. Therefore, G-CSF may be an effective method to treat thin endometrium. Several clinical studies have investigated the use of G-CSF to treat women with a thin endometrium [12, 13, 14, 15, 16]. However, inconsistent results have been reported with regards to the influence of intrauterine G-CSF infusion on pregnancy rate in clinical studies. Some studies have reported an improvement in pregnancy rate [12, 13, 14] while others did not [15, 16]. Therefore, in the present study, we conducted a meta-analysis to investigate the effect of G-GSF on thin endometrium during ART in the hope that this may provide useful guidance on how to prepare the endometrium for embryo transfer in patients with a thin endometrium.
(1) The style of the study was a randomized controlled trial (RCT) or non-RCT.
(2) Study subjects were patients whose endometrial thickness was
(1) The style of the study was an observational study, descriptive study, retrospective study, case report or review. (2) The study subjects were animals. (3) The baseline of the treatment group and the control group was inconsistent. (4) Studies involved a defective design or inappropriate statistical methods and could not be corrected. (5) Duplicate studies.
We searched PubMed, the Cochrane Library, Embase, Scopus and Web of Science from their inception to December 2022; we also conducted a manual search. The search strategy was as follows: ((“Reproductive Techniques, Assisted”[Mesh]) OR (assisted reproducti*) OR (IVF) OR (in vitro fertili*) OR (ICSI) OR (intracytoplasmic sperm injection) OR (embryo* AND transfer*) OR (blastocyst* AND transfer*) OR (FET) OR (ET) OR (Embryo Transfer) OR (ovarian stimulation) OR (ovulation induction) OR (controlled ovarian hyperstimulation) OR (COH)) AND ((thin* endometri*) OR (endometri* thin*) OR (endometri* thick*)) AND ((“Granulocyte Colony-Stimulating Factor”[Mesh]) OR (Granulocyte Colony Stimulating Factor) OR (GCSF) OR (neupogen) OR (filgrastim) OR (pegfilgrastim) OR (lenograstim) OR (molgramostim) OR (sargramostim)) (Appendix Table 3).
Two reviewers carried out articles selected and data extracted independently. First, the reviewers assessed relevant studies by reading the title and abstract. Then, they read through the entire study to decide whether it met the inclusion criteria. Next, the two reviewers compared their results; if they disagreed, they would take advice from an expert in the field who acted as a third reviewer. Finally, the reviewers acquired data from each selected study based on a pre-prepared data collection table. The corresponding author was contacted to obtain data whenever there was an incomplete dataset. The reviewers collated a range of data: (1) basic data, including the first author’s name and publication date; (2) study design; (3) factors used to assess the risk of bias; (4) essential patient characteristics, including the size of the treatment and control groups, nation, patient age and baseline endometrial thickness; (5) information relating to intervention strategies including the timing, method, dose and frequency of intrauterine G-CSF infusion; (6) outcome indicators, including clinical pregnancy rate, embryo implantation rate and endometrial thickness.
The meta-analysis was carried out with Review Manager 5.3 (Cochrane
Collaboration, Copenhagen, Denmark) [19]. We used mean differences (MD) to describe
continuous variables and relative risk (RR) to show categorical variables. We
calculated point estimates and 95% confidence intervals (95% CIs) for each
outcome measure. The meta-analysis was performed with random and fixed effects
models according to the I
Following careful selection and consideration of the inclusion and exclusion criteria, 8 studies were finally selected for the meta-analysis, including 6 RCTs [12, 13, 14, 20, 21, 22] and 2 non-RCTs [15, 16]. A total of 673 patients were included in our research. Fig. 1 shows the number of selected studies and the reasons for exclusion at each step.
 Fig. 1.
Fig. 1.Flow chart showing study selection.
The characteristics of the included studies are presented in Table 1 (Ref. [12, 13, 14, 15, 16, 20, 21, 22]), including the number of patients, nation, mean age, baseline endometrial thickness, interventions, and outcome measures for each study These studies covered 6 countries (2 in Iran, 2 in China, and each in Portugal, Spain, Poland, India). A total of 8 eligible studies were included in this meta-analysis. 5 studies reported the embryo implantation rate, 7 eligible studies reported the clinical pregnancy rate, 4 studies reported endometrial thickness.
| Study | Patients No. (T/C) | Mean age (T/C, years) | Baseline EM (mm) (T/C) | EM after intervention | Interventions (T/C) | Outcome measures |
|---|---|---|---|---|---|---|
| Singh R 2015 [12] | 48 (24/24) | 6.49 |
8.79 |
Intrauterine infusion of 300 µg/1 mL G-CSF on the day of hCG administration (second infusion following oocyte retrieval in subgroup patients) vs. intrauterine perfusion of placebo (1 mL saline solution) | IR, CPR | |
| Sarvi F 2017 [13] | 28 (15/13) | 31.6 |
4.1 |
9.1 |
Intrauterine infusion of 300 µg/1 mL G-CSF on the day of hCG administration; if EM was less than 6 mm, a second dose of G-CSF was injected 2–3 days after oocyte retrieval day in 3/15 patients vs. intrauterine perfusion of placebo (1 mL saline solution) | EM, IR, CPR |
| Singh R 2018 [14] | 112 (56/56) | NA | 6.23 |
8.46 |
Intrauterine infusion or subcutaneous injection of G-CSF 300 µg/1 mL on the day of hCG administration; a second dose of G-CSF after oocyte retrieval day in some patients vs. placebo (intrauterine perfusion of saline solution or subcutaneous injection of decavitamin) | IR, CPR |
| Eftekhar M 2014 [15] | 68 (34/34) | 30.81 |
5.63 |
7.91 |
Intrauterine infusion of 300 µg/1 mL G-CSF at the 12th–13th day of frozen-thawed embryo transfer cycle, a second dose of G-CSF was given 2–3 day after first infusion in 6/34 patients vs. blank control | EM, CPR |
| Kunicki M 2017 [16] | 62 (29/33) | 38 (32.75–41.25)/37 (35–40) | 6.50 (5.50–6.80)/6.40 (5.50–7.00) | 7.90 (6.58–8.70)/6.90 (6.0–7.75) | Intrauterine infusion of 300 µg/1 mL G-CSF at 9th day of frozen-thawed embryo transfer in endometrial preparation vs. blank control | IR, CPR |
| Xu B 2015 [20] | 66 (14/52) | 31.9 |
5.7 |
8.5 |
Intrauterine instillation of 300 µg G-CSF (100 µg/0.6 mL) on the day that one follicle became dominant (almost 12 × 12 mm in diameter) vs. blank control | IR, CPR |
| Jindal PC 2021 [21] | 60 (30/30) | NA | 5.9 |
7.9 |
Intrauterine infusion of 300 |
EM, CPR |
| Zhang Y 2022 [22] | 229 (114/115) | 31.7 |
5.50 |
7.91 |
Intrauterine erfusion with 300 mg (1.8 mL) G-CSF vs. 1.8 mL normal saline | EM |
Table footnotes: T, treatment group (G-CSF group); C, control group; hCG, human chorionic gonadotropin; NA, Not applicable; EM, endometrial thickness; IR, implantation rate; CPR, clinical pregnancy rate.
An assessment for the risk of bias for the included RCTs is shown in Figs. 2,3 RCTs [13, 20, 22] applied a random sequence generation process to avoid selection bias; one of these RCTs [13] also described the use of a ‘sealed envelope’ to ensure allocation concealment. 4 RCTs [12, 13, 14, 22] described the blinding of participants and personnel, and 5 RCTs [12, 13, 14, 21, 22] described the blinding of outcome assessments. However, 2 RCTs [12, 14] had an incomplete dataset for outcomes. 6 RCTs [12, 13, 14, 20, 21, 22] did not describe selective reporting and other forms of bias. All RCTs were considered to represent medium risk.
 Fig. 2.
Fig. 2.Assessment of risk of bias for the included RCTs.
 Fig. 3.
Fig. 3.Comparison of embryo implantation rate between G-CSF group and control group.
Table 2 (Ref. [15, 16]) shows the results derived from the assessment of methodological quality of non-RCTs. Analysis showed that the non-RCTs scored 22 (more than 12 points) and were considered to represent a low risk of bias.
| Study | Non-comparative study | Score | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A clearly stated aim | Inclusion of consecutive patients | Prospective collection of data | Endpoints appropriate to the aim of the study | Unbiased assessment of the study endpoint | Follow-up period appropriate to the aim of the study | Loss to follow-up less than 5% | Prospective calculation of the study size | ||
| Eftekhar M 2014 [15] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | / |
| Kunicki M 2017 [16] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | / |
| Study | Additional criteria in the case of comparative study | Score | |||||||
| An adequate control group | Contemporary groups | Baseline equivalence of groups | Adequate statistical analyses | ||||||
| Eftekhar M 2014 [15] | 2 | 2 | 2 | 2 | 22 | ||||
| Kunicki M 2017 [16] | 2 | 2 | 2 | 2 | 22 | ||||
Table footnotes: The items are scored 0 (not reported), 1 (reported but inadequate) or 2 (reported and adequate).
A total of 5 studies reported the embryo implantation rate [12, 13, 14, 16, 20]. A
fixed-effects model was employed when statistical results were homogeneous
(p = 0.87, I
 Fig. 4.
Fig. 4.Comparison of embryo implantation rate between G-CSF group and control group in RCTs.
All 7 eligible studies were involved when considering clinical pregnancy rate
[12, 13, 14, 15, 16, 20, 21]. We used a fixed-effect model because there was adequate
homogeneity among studies (p = 0.81, I
 Fig. 5.
Fig. 5.Comparison of clinical pregnancy rate between G-CSF group and control group.
 Fig. 6.
Fig. 6.Comparison of clinical pregnancy rate between G-CSF group and control group in RCTs.
Four studies were included when considering endometrial thickness [13, 15, 21, 22]. A random-effect model was adopted due to statistical heterogeneity across
these studies (p
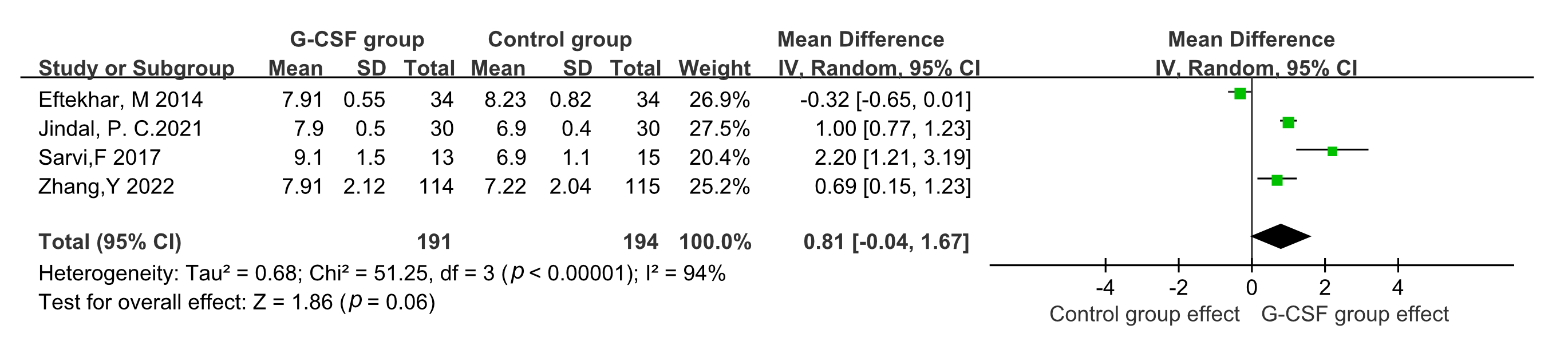 Fig. 7.
Fig. 7.Comparison of endometrial thickness between G-CSF group and control group.
 Fig. 8.
Fig. 8.Comparison of endometrial thickness between G-CSF group and control group in RCTs.
Successful assisted reproduction requires a receptive endometrium; however, studies have shown that the implantation rate is significantly lower in women with a thin endometrium [23]. A thin endometrium has an unfavorable effect on reproductive outcome. Various therapeutic methods have been used for patients with a thin endometrium. Although there are many types of treatments, most existing methods only achieve a slight change in endometrial thickness and pregnancy outcome. Consequently, it is very challenging to treat women with a thin endometrium when considering ART [24]. Previous studies have suggested that G-CSF may increase the embryo implantation rate and clinical pregnancy rate in women with a thin endometrium. G-CSF, as a type of cytokine, can affect bone marrow mesenchymal stem cells and decidual macrophages, thus influencing endometrial growth and development [25]. Using a rat model of thin endometrium, a previous study showed that the intrauterine infusion of G-CSF could improve endometrial receptivity by regulating endometrial hyperplasia and angiogenesis [26]. Zhao et al. [27] reported that G-CSF could mobilize stem cells to the site of injury and repair tissue in animal models, when compared with control groups, G-CSF resulted in obvious thickening of the endometrium along with increased expression levels of cytokeratin and vimentin. The intrauterine perfusion of G-CSF, as a chemical and mechanical stimulus, is considered to induce the secretion of endogenous cytokines and activate endocrine-paracrine pathways, thus leading to successful embryo implantation and pregnancy [15]. At the genetic level, G-CSF can downregulate the expression of hsa_circ_0001550, the downstream target genes of hsa_circ_0001550 participate in regulating endometrial receptivity and embryo implantation, so G-CSF may affect embryo implantation by adjusting hsa_circ_0001550-miRNAmRNA interaction network [28]. Furthermore, by promoting embryonic adhesion, cell migration, tissue remodeling, and the expression of angiogenesis-related genes, G-CSF may contribute to embryo implantation [29]. G-CSF has been shown to be involved in embryonic growth and development [30] and play a role in the maintenance of pregnancy [31].
Several clinical studies have investigated the use of G-CSF to treat women with a thin endometrium with G-CSF. In 2011, Gleicher et al. [30] were the first to report four infertile women with a thin endometrium who successfully became pregnant by ART following the intrauterine perfusion of G-CSF. Subsequently, the same research group recruited another cohort of patients with a persistent thin endometrium who had failed conventional treatment and underwent intrauterine G-CSF perfusion. These researchers found that these patients achieved a low but reasonable clinical pregnancy rate following the intrauterine infusion of G-CSF [32]. Since then, several studies have investigated the use of G-CSF to treat women with a thin endometrium. However, the results of these studies were inconsistent. For example, Mishra et al. [33] reported that G-CSF led to a slight increase in endometrial thickness in women with a persistent thin endometrium; however, G-CSF did not improve pregnancy rate in such patients. In another study, Swati et al. [34] found that G-CSF improved endometrial thickness only in about a third of women with a persistent thin endometrium. Kim et al. [35] reported that G-CSF improved the thickness of a thin endometrium without causing intrauterine adhesions and increased the chances of conception and embryo implantation. Due to such inconsistency, it was evident that we needed to perform a meta-analysis of the published studies to determine whether G-CSF can affect endometrial thickness and pregnancy.
In total, we investigated 8 studies (consisting of 6 RCTs and 2 non-RCTs including 673 patients) in this meta-analysis. We discovered that intrauterine infusion or the subcutaneous injection of G-CSF significantly increased embryo implantation and clinical pregnancy rates when compared with controls. The effect of G-CSF on clinical pregnancy rate and embryo implantation rate were consistent before and after subgroup analysis, irrespective of whether we were considering RCTs or not. The use of G-CSF did not have a significant effect on endometrial thickness. Three studies were used to analyze endometrial thickness, including two RCTs and one non-RCT. Based on the results of sub-group analysis for two RCT studies, we found that G-CSF did increase endometrial thickness. As we know women with thin endometrium may have a higher risk of preterm birth, low birth weight, and miscarriage, embryo transplantation in patients with thin endometrium should be cautious, delaying transplantation to the cycle of thickening before transplantation can be suggested [36]. Therefore, G-CSF may improve the pregnancy outcome by increasing the thickness of endometrium. However, only two RCTs were involved in this analysis; consequently, there is a significant need for more RCTs to fully validate our findings.
A systematic review and meta-analysis of the use of G-CSF in ART was previously
published by Kamath et al. [37] in 2017; however, this study only
included 1 non-RCTs and 3 RCTs with low quality evidence. In the present
meta-analysis, we included 8 studies, including 6 RCTs and 2 non-RCTs; there has
been an increase in relevant research over recent years and the level of evidence
for the studies included in our study was better than the previous study [37].
These previous authors reported significantly a higher clinical pregnancy rate
after the use of G-CSF when compared with controls
[RR = 2.43, 95% CI (1.09,
5.40), I
There are several limitations to our current analysis that need to be considered. First, the sample size of our analysis was small, as only five RCTs and two non-RCTs were included; this may have led to bias. Despite the limited number of studies, when stratified by RCTs vs. non-RCTs, subgroup analyses related to embryo implantation rate and clinical pregnancy rate showed consistent results with those of the overall pooled analyses. Inconsistent results were detected with regards to the influence of G-CSF on endometrium thickness; therefore, further studies are now needed to clarify the true benefits of G-CSF treatment. Second, the dosage and methods of G-CSF administration, as well as the underlying causes of thin endometrium, tended to vary across the included studies. Since the available studies did not individualize treatment strategies according to different underlying causes of thin endometrium, it was impossible to generate a definitive conclusion with this dataset. Therefore, well-designed randomized controlled studies with a large sample size and high quality are still needed to validate our findings. Current studies are still in the exploratory stage and there is no uniform standard for dosage, the times of administration, route of administration and timing of administration.
The results of our meta-analysis indicate that G-CSF may increase embryo implantation rate and clinical pregnancy rate of patients with thin endometrium during ART, G-CSF may have the potential to increase the endometrial thickness, although further studies are needed to fully validate the impact of G-CSF on endometrial thickness. Nevertheless, our findings should be considered cautiously conclusion owing to the limited number and quality of the studies involved in our meta-analysis, as well as differences in the etiology of thin endometrium, the frequency and route of G-CSF administration. Large-scale, high-quality, multi-center RCTs are now needed to fully elucidate the benefits of G-CSF on thin endometrium.
HC, L-JL and GC designed the study. L-JL, GC carried out the research and collated data. L-ZX interpreted the data of the study and supervised L-JL. All authors wrote and edited the manuscript and approved the final version. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
We appreciate Linbo-Gao who provided support during the writing of this manuscript.
This meta-analysis formed part of a ‘Study on the risk factor assessment tool for premature ovarian failure’ which is funded by the Technological Innovation and Development Program of Chengdu Bureau of Science and Technology (2019-YFYF-00101-SN); and was also part of ‘Creating a risk factor assessment tool for premature ovarian failure’, which was funded by the Key Science Research Program of Health Commission of Sichuan Province (20ZD008).
The authors declare no conflict of interest.
See Table 3.
| Cochranelibrary | ||
|---|---|---|
| #1 MeSH descriptor: [Reproductive Techniques, Assisted] explode all trees MeSH | 3583 | |
| #2(assisted reproducti*) OR (IVF) OR (in vitro fertili*) OR (in-vitro fertil*) OR (ICSI) OR (intracytoplasmic sperm injection) OR ((embryo* AND transfer*)) OR ((blastocyst* AND transfer*)) OR (FET) OR (ET) OR (Embryo Transfer) OR (ovarian stimulation) OR (ovulation induction) OR (controlled ovarian hyperstimulation) OR (COH) | 110021 | |
| #3 #2 OR #3 | 110172 | |
| #4 (thin* endometri*) OR (endometri* thin*) OR (endometri* thick*) | 2664 | |
| #5 MeSH descriptor: [Granulocyte Colony-Stimulating Factor] explode all trees MeSH | 1559 | |
| #6 (Granulocyte Colony Stimulating Factor) OR (G-CSF) OR (GCSF) OR (neupogen) OR (filgrastim) OR (pegfilgrastim) OR (lenograstim) OR (molgramostim) OR (sargramostim) | 7087 | |
| #7 #5 OR #6 | 7087 | |
| #8 #1 AND #6 AND #7 | 61 | |
| Embase | ||
| #1 ‘reproductive techniques, assisted’/exp OR ‘reproductive techniques, assisted’ OR (assisted AND reproducti*) OR ‘ivf’/exp OR ivf OR ‘in vitro’/exp OR ‘in vitro’ OR (in AND vitro AND fertili*) OR ‘icsi’/exp OR icsi OR ‘intracytoplasmic sperm injection’/exp OR ‘intracytoplasmic sperm injection’ OR (intracytoplasmic AND (‘sperm’/exp OR sperm) AND (‘injection’/exp OR injection)) OR (embryo* AND transfer*) OR (blastocyst* AND transfer*) OR fet OR et OR ‘embryo transfer’/exp OR ‘embryo transfer’ OR ((‘embryo’/exp OR embryo) AND (‘transfer’/exp OR transfer)) OR ‘ovarian stimulation’/exp OR ‘ovarian stimulation’ OR (ovarian AND (‘stimulation’/exp OR stimulation)) OR ‘ovulation induction’/exp OR ‘ovulation induction’ OR ((‘ovulation’/exp OR ovulation) AND (‘induction’/exp OR induction)) OR ‘controlled ovarian hyperstimulation’/exp OR ‘controlled ovarian hyperstimulation’ OR (controlled AND ovarian AND (‘hyperstimulation’/exp OR hyperstimulation)) OR coh | 9389150 | |
| #2 ‘granulocyte colony-stimulating factor’/exp OR ‘granulocyte colony-stimulating factor’ OR ‘granulocyte colony stimulating factor’/exp OR ‘granulocyte colony stimulating factor’ OR ((‘granulocyte’/exp OR granulocyte) AND (‘colony’/exp OR colony) AND stimulating AND factor) OR gcsf OR ‘neupogen’/exp OR neupogen OR ‘filgrastim’/exp OR filgrastim OR ‘pegfilgrastim’/exp OR pegfilgrastim OR ‘lenograstim’/exp OR lenograstim OR ‘molgramostim’/exp OR molgramostim OR ‘sargramostim’/exp OR sargramostim | 117670 | |
| #3 thin* AND endometri* OR (endometri* AND thin*) OR (endometri* AND thick*) | 12997 | |
| #4 #1 AND #2 AND #3 | 140 | |
| PubMed | ||
| #1 “Reproductive Techniques, Assisted”[Mesh] OR (assisted reproducti*) OR (IVF) OR (in vitro fertili*) OR (ICSI) OR (intracytoplasmic sperm injection) OR (embryo* AND transfer*) OR (blastocyst* AND transfer*) OR (FET) OR (ET) OR (Embryo Transfer) OR (ovarian stimulation) OR (ovulation induction) OR (controlled ovarian hyperstimulation) OR (COH) | 10586096 | |
| #2 (thin* endometri*) OR (endometri* thin*) OR (endometri* thick*) | 6033 | |
| #3 (“Granulocyte Colony-Stimulating Factor”[Mesh]) OR (Granulocyte Colony Stimulating Factor) OR (G-CSF)) OR (GCSF) OR (neupogen) OR (filgrastim) OR (pegfilgrastim) OR (lenograstim) OR (molgramostim) OR (sargramostim) | 4,7057 | |
| #4 #1 AND #2 AND #3 | 46 | |
| Web of Science | ||
| #1 TS=(Reproductive Techniques, Assisted) OR ALL=(assisted reproducti*) OR ALL=(IVF) OR ALL=(in vitro fertili*) OR ALL=(in-vitro fertil*) OR ALL=(ICSI) OR ALL=(intracytoplasmic sperm injection) OR ALL=((embryo* AND transfer*)) OR ALL=((blastocyst* AND transfer*)) OR ALL=(FET) OR ALL=(ET) OR ALL=(Embryo Transfer) OR ALL=(ovarian stimulation) OR ALL=(ovulation induction) OR ALL=(controlled ovarian hyperstimulation) OR ALL=(COH) | 1425950 | |
| #2 ALL=(thin* endometri*) OR ALL=(endometri* thin*) OR ALL=(endometri* thick*) | 8277 | |
| #3 TS=(Granulocyte Colony-Stimulating Factor) OR ALL=(Granulocyte Colony Stimulating Factor) OR ALL=(G-CSF) OR ALL=(GCSF) OR ALL=(neupogen) OR ALL=(filgrastim) OR ALL=(pegfilgrastim) OR ALL=(lenograstim) OR ALL=(molgramostim) OR ALL=(sargramostim) | 70291 | |
| #4 #3 AND #2 AND #1 | 67 | |
| Scopus | ||
| #1 TITLE-ABS-KEY(“Reproductive Techniques, Assisted” OR (assisted AND reproducti* ) OR (ivf) OR (in AND vitro ANDfertili*) OR (icsi) OR (intracytoplasmic AND sperm AND injection) OR (embryo* AND transfer*) OR (blastocyst* AND transfer*) OR (fet) OR (et) OR (embryo AND transfer) OR (ovarian AND stimulation) OR (ovulation AND induction) OR (controlled AND ovarian AND hyperstimulation) OR (coh) | 1888943 | |
| #2 TITLE-ABS-KEY (thin* endometri*) OR ALL=(endometri* thin*) OR ALL=(endometri* thick*) | 9468 | |
| #3 TITLE-ABS-KEY(“Granulocyte Colony-Stimulating Factor” OR (granulocyte ANDcolony AND stimulating AND factor) OR (gcsf) OR (neupogen) OR (filgrastim) OR (pegfilgrastim) OR (lenograstim) OR (molgramostim) OR (sargramostim)) | 99495 | |
| #4 #3 AND #2 AND #1 | 70 | |
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
