- Academic Editor
†These authors contributed equally.
Background: We aim to determine the
relationship between intra-amniotic sludge and the amniotic fluid proteome in
cases of acute cervical insufficiency. Methods: This was a secondary
analysis of data from an existing prospective cohort of women with acute cervical
insufficiency. Amniotic fluid had previously been analyzed by Gram stain, culture
and proteomics perioperatively. Post-processing review of stored ultrasound
images to evaluate for the presence of intra-amniotic sludge (IAS) was performed
by two blinded and independent observers. Results, lab, clinical, proteomic and
outcome data were compared between groups with and without sludge.
Results: Ten participants with acute cervical insufficiency from the
initial cohort were included [IAS group (n = 4) and non-IAS group (n = 6)]. 75%
of participants with sludge had negative Gram stain and culture compared to 17%
amongst cases without sludge. 18 proteins (3.3%) were differentially abundant
between the 2 groups (p
Preterm birth remains the leading cause of perinatal mortality and morbidity worldwide [1]. Cervical insufficiency (CI) is an important cause of second trimester loss and periviable preterm birth [2]. Cervical insufficiency is defined as painless shortening and premature dilatation of the cervix in the second trimester and without intervention, is invariably a precursor to delivery at a time when neonatal outcomes are poor [3, 4]. Mechanical treatments for cervical insufficiency include surgical cerclage and cervical pessary [5, 6]. Surgical placement of a cervical cerclage aims to close the cervix using suture with the intent of prolonging the pregnancy and improving fetal outcome [2, 3]. However, emergency cerclage placement is potentially dangerous if there is underlying infection of the amniotic fluid or introduction of bacteria when the exposed membranes are pushed back into the uterus [3, 7, 8, 9, 10]. In either case, emergency cervical cerclage is contraindicated in the presence of intra-amniotic infection as it carries a significant risk of maternal sepsis and associated sequelae (including death), as well as poor neonatal outcome [11, 12, 13, 14, 15, 16]. It is therefore essential to rule out infection before consideration of surgical intervention [10].
Traditionally, amniocentesis with Gram stain and bacterial culture of the amniotic fluid is performed prior to placement of emergency cerclage to rule out infection; however, these methods lack sensitivity and as an invasive procedure, can itself be associated with risk [17, 18]. In an attempt to improve our ability to detect subclinical intra-amniotic infection, multiple biomarkers in the amniotic fluid, cervicovaginal secretions and maternal serum have been extensively studied in the literature. Although correlation with intra-amniotic infection has been demonstrated, widespread use in the clinical setting has been limited by their sensitivity and specificity [18, 19, 20]. Mass spectrometry-based proteomics of the amniotic fluid and cervicovaginal secretions have identified differential protein expression in cases of preterm labour and premature rupture of membranes. These biomarkers and/or immune signatures may be indicative of intra-amniotic infection [21, 22, 23, 24, 25]. Mass spectrometry-based proteomics is used to measure numerous protein biomarkers simultaneously, offering a discovery platform to identify mechanisms of pathogenesis [26, 27, 28, 29]. We have recently demonstrated that in women with cervical insufficiency, proteomic profiles tend to differ in women who deliver more or less than one week after their diagnosis of cervical insufficiency [30]. A recent retrospective cohort of women with cervical insufficiency and subsequent placement of emergency cerclage identified 68 proteins that were differentially expressed in women who subsequently had a spontaneous preterm birth less than 34 weeks [21]. Although this technology is in its discovery phase, proteomic analysis of amniotic fluid may provide additional insight into the complex mechanism of intra-uterine inflammation and infection.
Whether for traditional bacterial culture or proteomic analysis, sampling of the amniotic fluid via amniocentesis continues to carry inherent risks of invasive testing including pregnancy loss, preterm birth and iatrogenic infection. An ideal test prior to placing a cervical cerclage would be both sensitive and non-invasive. As such, researchers are actively searching for non-invasive markers of intra-amniotic infection, including the sonographic detection of intra-amniotic sludge. Intra-amniotic sludge (IAS) is a sonographic finding of free-floating, hyperechoic material in the amniotic fluid, typically present by the internal os of the cervix on transvaginal ultrasound (Fig. 1) [31]. IAS has been associated with intra-amniotic infection, histological chorioamnionitis and preterm birth [32, 33, 34, 35, 36, 37]. Bacteria and fungi have been isolated from IAS collected both transvaginally and transabdominally and the presence of biofilms within those samples has been demonstrated [19, 31, 36, 38]. Historically, the presence of IAS has been largely unrecognized as a potential predictor of adverse perinatal outcomes. The existing literature is inconclusive regarding the ability of IAS to reliably predict the development of overt chorioamnionitis and other pregnancy-related complications [39].
 Fig. 1.
Fig. 1.2D greyscale ultrasound images of intra-amniotic sludge (IAS). IAS (circled) as seen on transabdominal (A) and transvaginal (B) ultrasound.
Herein, we present the first study interrogating the relationship between the amniotic fluid proteome and sonographic appearance of IAS. The objective of this study was to determine whether there are associations between the presence of IAS and the amniotic fluid proteomic profile that influence clinical outcomes amongst women with acute cervical insufficiency.
This was a pilot study involving a secondary analysis of a prospective cohort of women presenting with acute cervical insufficiency [30]. From the original cohort of 40 women, patients diagnosed with acute cervical insufficiency considering emergency cerclage were eligible and included in this analysis. Acute cervical insufficiency was defined as interval cervical shortening on serial ultrasound of either: (i) less than 1.5 cm intact length; or (ii) less than 2.5 cm with funneling, dilation and/or membrane prolapse. Cases were further subclassified according to presence/absence of membrane prolapse (high versus low grade). High grade cervical insufficiency was defined as evidence of an open cervix with visible fetal membranes at or below the external os. Low grade cervical insufficiency was defined as evidence of cervical shortening with/without membrane funneling above the level of external os. All those included had amniotic fluid samples collected prospectively via perioperative amniocentesis. Samples were collected from November 2014 to August 2016 at the Women’s Hospital Fetal Assessment Unit in Winnipeg, Manitoba, Canada. All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of University of Manitoba (approval number: H2019:481 (HS23469), linked to H2014:328). Written patient consent was also obtained for inclusion of the ultrasound images included in this manuscript.
Sample collection has been previously described [30]. All transabdominal
amniocenteses were performed following the diagnosis of cervical insufficiency
under sterile technique and ultrasound guidance by a single maternal-fetal
medicine specialist. Three additional millilitres of amniotic fluid was withdrawn
from the amniotic sac with a 22–25 gauge needle for research purposes. Gram
stain, aerobic and anaerobic bacterial cultures were performed by the hospital’s
clinical microbiology laboratory. Proteomic analysis has been previously
described [30]. Briefly, 75
Post-processing review of stored ultrasound images taken at the time of diagnosis of cervical insufficiency was performed by two blinded observers to evaluate for the presence or absence of IAS. Sludge was considered present when dense particulate matter was visualized in proximity to the internal cervical os on transvaginal ultrasound, consistent with previously published studies (Fig. 1A,B). Interobserver discordance was resolved by consensus opinion.
Descriptive statistics were used to evaluate maternal characteristics and pregnancy outcomes. Continuous variables were presented as medians with ranges while dichotomous and categorical variables were described as proportions. Clinical data was analysed using Mann-Whitney U-tests and Fisher’s Exact or Chi-squared tests using GraphPad Prism v7.02 (GraphPad Software Inc., La Jolla, CA, USA), and any missingness in the data formally explored using sensitivity analyses when indicated. We chose to evaluate proteins that differed between IAS and non-IAS groups with a p-value below 0.07 using a Mann-Whitney U-test. Analyses were conducted in R v3.6.2 (R Foundation for Statistical Computing, Vienna, Austria). Principal component analysis was conducted using proteins differentially abundant between study groups in MatLab (MathWorks, R2015b, Natick, MA, USA) and EigenVector software (Eigenvector Research, Inc., Wenatchee, WA, USA). Hierarchical clustering was performed on proteins found to be differentially abundant between study groups using complete linkage and Pearson correlation as the distance metric via the NMF package (v0.21.0) available in R (v3.6.2). Protein functional and pathway analysis was conducted using ConsensusPathDB (Max Planck Institute for Molecular Genetics) (http://cpdb.molgen.mpg.de/) and Ingenuity Pathway Analysis (Qiagen). For the proteomic analyses, p values were calculated using hypergeometric tests and right-tailed Fisher’s Exact tests.
From the original cohort of 40 women, 10 women met inclusion criteria and were included in the analysis. The median age was 32 years (range 22–40), median gravidity of 3 (1–10), median parity of 1 (0–6), and a median gestational age of 21 + 6 weeks (19 + 1 to 23 + 3) at enrolment. Four women experienced greater than one week of latency (period of time between diagnosis of CI and delivery) while six had a latency period of less than one week. Four of the ten women delivered beyond viability (greater than 24 weeks gestation).
Four participants had evidence of IAS detected on transvaginal ultrasound at the time of diagnosis of cervical insufficiency (IAS group) while six participants had no evidence of IAS on ultrasound (non-IAS group). There were no major differences in maternal age, gravidity, parity, gestational age at time of diagnosis, grade of cervical insufficiency, Gram stain results, period of latency and fetal outcomes between IAS and non-IAS groups (Table 1). Two participants did not have targeted transvaginal images available, but review of available images did not detect features of particulate matter in the amniotic fluid or evidence of IAS on specific lower segment views of the internal cervical os on transabdominal ultrasound and they were included in the non-IAS group. The third case did not have available images to accompany the pre-cerclage ultrasound report. Consensus for including this case in the non-IAS group was reached as evidence of sludge would likely have been documented would it have been present. Following sensitivity analysis which showed no difference amongst these cases, a total of six participants were therefore included in the non-IAS group. Overall, only three participants had positive Gram stain results upon sampling with amniocentesis. Three of four participants with IAS (75%) had negative Gram stains, in contrast with only two patients (33%) in the non-IAS group. In the IAS group, three of the four (75%) reached viability to compared to only one of the six (17%) participants without IAS (Table 1).
| Variable | Category | Amniotic sludge | |
|---|---|---|---|
| No (n = 6) | Yes (n = 4) | ||
| Median mother’s age (range) | 29 (26–35) | 35 (22–40) | |
| Median gestational age in days (range) | 147 (134–164) | 154 (147–159) | |
| Median gravidity (range) | 4 (1–10) | 3 (2–8) | |
| Median parity (range) | 3 (0–6) | 1 (0–3) | |
| Incompetency grade, n (%) | High | 2 (33%) | 2 (50%) |
| Low | 2 (33%) | 1 (25%) | |
| Not specified | 3 (33%) | 1 (25%) | |
| Gram stain, n (%) | Positive | 3 (50%) | 0 (%) |
| Negative | 2 (34%) | 3 (75%) | |
| Not specified | 1 (18%) | 1 (25%) | |
| Latency, n (%) | Yes | 1 (17%) | 3 (75%) |
| No | 5 (83%) | 1 (25%) | |
| Fetal outcome, n (%) | Viable | 1 (17%) | 3 (75%) |
| Non-viable | 5 (83%) | 1 (25%) | |
A secondary analysis of previously acquired mass spectrometry data was conducted
to determine possible protein relationships amongst the groups with and without
IAS. Of the 551 proteins commonly measured amongst the ten amniotic fluid
samples, 18 (3.3%) proteins differed (Mann-Whitney, p
| Gene name | Protein name | p-value | Mean non-IAS group | Mean IAS group | Fold change difference (IAS-nonIAS) | Functions |
|---|---|---|---|---|---|---|
| LBP | Lipopolysaccharide-binding protein | 0.00952 | 0.10 | –0.83 | –0.94 | Defense response |
| CD81 | CD81 antigen | 0.00952 | –0.17 | 0.24 | 0.74 | Immunological synapse formation |
| IGHV4-34 | Immunoglobulin heavy variable 4-34 | 0.0191 | 0.36 | –0.62 | –0.99 | Defense response |
| CRNN | Cornulin | 0.0191 | –0.067 | 0.53 | 0.60 | Cell-cell adhesion |
| FBLN1 | Fibulin-1 | 0.0191 | –0.0075 | 0.72 | 0.73 | Defense response |
| HMGN1 | Non-histone chromosomal protein HMG-14 | 0.0381 | 1.12 | 0.024 | –1.09 | Chromatin organization |
| CFB | Complement factor B | 0.0381 | 0.50 | 0.001 | –0.50 | Complement activation, inflammatory response |
| SERPINC1 | Antithrombin-III | 0.0381 | 0.25 | –0.02 | –0.27 | Acute inflammatory response |
| EVPL | Envoplakin | 0.0381 | –0.035 | 0.45 | 0.49 | Cornification, wound healing |
| EPS8L1 | Epidermal growth factor receptor kinase substrate 8-like protein 1 | 0.0381 | –1.36 | 0.73 | 2.09 | Positive regulation of ruffle assembly |
| HBG2 | Hemoglobin subunit gamma-2 | 0.0381 | –1.27 | 0.94 | 2.21 | Oxygen binding |
| PRDX5 | Peroxiredoxin-5, mitochondrial | 0.067 | 0.63 | –0.06 | –0.69 | Response to oxidative stress |
| FGA | Fibrinogen alpha chain | 0.067 | 0.35 | –0.18 | –0.52 | Immune response |
| SCGB3A2 | Secretoglobin family 3A member 2 | 0.067 | 0.091 | 0.54 | 0.45 | Anti-inflammatory |
| PPL | Periplakin | 0.067 | 0.077 | 0.66 | 0.58 | Cornification, wound healing |
| AHNAK | Neuroblast differentiation-associated protein AHNAK | 0.067 | –0.26 | 0.35 | 0.61 | Regulation of RNA splicing |
| MYDGF | Myeloid-derived growth factor | 0.067 | 0.05 | 0.77 | 0.72 | Angiogenesis, apoptosis |
| KRT6A | Keratin, type II cytoskeletal 6A | 0.067 | –0.78 | 0.33 | 1.11 | Cornification, wound healing |
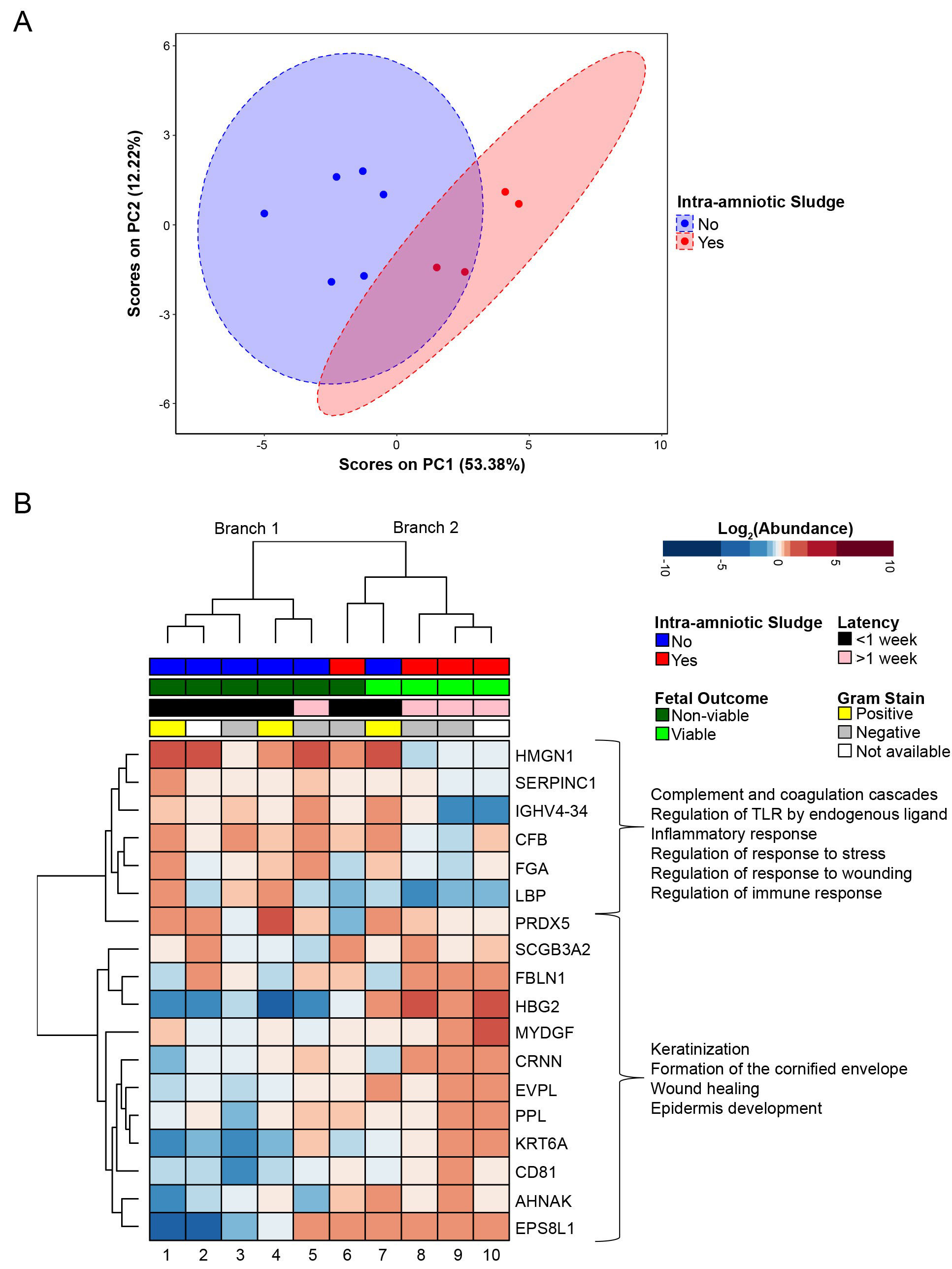 Fig. 2.
Fig. 2.Amniotic fluid protein differences between participants with and
without intra-amniotic sludge (IAS). (A) Principal component analysis of
proteins differentially abundant between IAS and non-IAS groups (p
Of the five samples that clustered to the right (branch 2), three participants
had greater than 1 week of latency. All four participants that had viable fetal
outcomes also clustered to the right (branch 2). Proteins increased in the right
(branch 2) cluster included keratinization (n = 3, p = 8.45
This exploratory analysis of the relationship of IAS and the amniotic fluid proteome in women diagnosed with acute cervical insufficiency suggests novel differences exist between those with or without ultrasound evidence of sludge. Keratinization, epidermis development and wound healing proteins were increased amongst cases with IAS. When IAS was not identified, inflammatory, stress and immune response signatures were increased while keratinization proteins were decreased. Other studies have identified biomarkers in women with acute cervical insufficiency associated with latency after emergency cervical cerclage such as increased proinflammatory cytokines, increased neutrophil-lymphocyte ratios, and increased neutrophil protein levels (elastase, defensins-1 and -2, S100-A8 S100A8, S100A9 and S100A12) [22, 40, 41, 42, 43]. Is it important to distinguish that these studies analyzed pre-selected biomarkers which have never been proven to be sufficiently specific or sensitive for routine use in clinical practice. To our knowledge, this is the first study to compare intra-amniotic sludge to the amniotic fluid proteome.
Very few studies have analyzed amniotic fluid using shotgun mass spectrometry-based proteomics from patients diagnosed with acute cervical insufficiency. In contrast to pre-selected biomarkers, this technique enables simultaneous identification of multiple proteins which more accurately captures the complex, multifactorial signatures associated with acute CI. Lee et al. [21] identified protein signatures that were associated with preterm birth before 34 weeks gestation in women with CI. These signatures included elevated phagocyte/neutrophil proteins (Myeloperoxidase (MPO), Neutrophil gelatinase-associated lipocalin (LCN2), Plastin-2 (LCP1), Lactotransferrin(LTF)). Interestingly, our initial analysis of women with CI included in this study found that women who experienced less than one week of latency had elevated defense response proteins including a number of the aforementioned phagocyte/neutrophil proteins (LCP1, Neutrophil defensin 3 (DEFA3), LCN2, LTF, S100A9) [30]. In our secondary analysis, proteins that were differentially abundant in women with and without IAS differed: patients with IAS and the most prolonged latency had lower levels of defense response proteins including lipopolysaccharide-binding protein (LBP), fibrinogen alpha chain (FGA), and complement factor B (CFB). This finding highlights a potential clinical role in detection of decreased inflammatory proteins for more accurately predicting prolonged latency in women with acute cervical insufficiency.
IAS has been identified as an independent risk factor for preterm prelabor rupture of membranes (PPROM), preterm delivery and histological chorioamnionitis both in cases of cervical insufficiency and in pregnancies at high risk of preterm birth without cervical insufficiency [32, 33, 34, 35, 36, 37]. Although IAS has been cultured and shown to incorporate bacteria, fungi and biofilms [32, 38], debate exists regarding whether all IAS seen on ultrasound truly represents intrauterine infection. Our findings suggest that IAS may represent indolent intrauterine infection or infection caused by organisms with the ability to create biofilms and thus attenuate the innate inflammatory response. This has been seen in infectious processes elsewhere in the body [44, 45]. Our results echo those of a recent retrospective cohort by Yoneda et al. [46] that demonstrated that patients with preterm labour and intact membranes had a shorter latency period when a pathogen was identified in the amniotic fluid via molecular techniques and no evidence of IAS when compared to cases with no identified pathogen in the amniotic fluid but with evidence of IAS. Although molecular techniques have been shown to be more sensitive than traditional culture, this requires targeted testing for identifying a specific pathogen. In addition, it is possible that sampling the upper compartment of the uterus via amniocentesis may be insufficient in certain cases of cervical insufficiency, especially if IAS suggests a contained infection in the lower segment of the uterus. At a minimum, amniocentesis simply done to culture an organism, by any testing modality, will uniformly miss other signs of intraamniotic inflammation.
Placement of an emergency cerclage in the setting of negative culture does not eliminate risk for the mother and fetus. Meticulous surveillance is required to avoid maternal morbidity. It is well established that although prematurity is the most significant fetal complication in cases of cervical insufficiency, the delivery of a fetus in the setting of chorioamnionitis worsens prognosis. Intrauterine infection has been associated with multiple long-term neurodevelopmental complications including cerebral palsy.
A growing body of evidence suggests that proteomic analysis provides valuable
information in the setting of cervical insufficiency [21, 22]. We have previously
shown that proteomic profiles differ in women who deliver more or less than one
week after diagnosis of cervical insufficiency [30]. In our small sample, protein
signatures associated with IAS were more prevalent in cases with prolonged
latency
Our small study has a number of strengths. This cohort represents a retrospective secondary analysis of prospectively collected amniotic fluid samples. Our cohort included women with acute cervical insufficiency, four of which (40%) had an advanced presentation with an open cervix and amniotic membranes funnelling to or beyond the external cervical os. The findings of this study were not influenced by cases of short cervical length that may have gone to term without intervention. In addition, no cases of prophylactic cerclages were included in our study. Prophylactic cerclages and cases of short cervical length are a different population and should not be included in a study to determine the role of intrauterine infection in acute cervical insufficiency.
Our study also has limitations that warrant discussion. The main limitation of
this study is its sample size as cases of cervical insufficiency are uncommon,
and those with prospectively collected amniotic fluid, even fewer. Due to the
small sample we were unable to control for maternal factors that may confound
results (e.g., maternal age, obstetrical history, smoking history or cervical
status). For the proteomic analyses, the p-value was relaxed to p
In conclusion, we demonstrated that mass spectrometry-based proteomics of amniotic fluid and the assessment of the presence of IAS at the time of diagnosis of cervical insufficiency are feasible. Patterns of protein expression in IAS cases demonstrated a predominance in functions of keratinization, epidermis development and wound healing, while cases without IAS demonstrated a heightened inflammatory pattern with increased stress and immune response signatures. The exploratory nature of this study illustrates the urgent need for large prospective studies to determine the utility of IAS and proteomics in the risk stratification of cases of cervical insufficiency and prediction of severe maternal and fetal morbidity and mortality. Further research will be important to determine whether the assessment of IAS and proteomic analysis of amniotic fluid could optimize the management of patients with acute cervical insufficiency.
Data may be available upon reasonable request.
RNMG, KDB, ADB, VP, SMM were involved with recruitment, collection of amniotic fluid samples, and specimen processing in the original cohort. RNMG and CLP were involved with ultrasound data collection and data management. Statistical analysis was performed by KDB. RNMG and CLP produced the initial drafts and final version of the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity.
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the University of Manitoba (approval number: H2019:481 (HS23469)).
We would like to thank Katarina Nikel for her support or the project, the team at the Women’s Hospital Fetal Assessment Unit in Winnipeg, Manitoba and the team from the Garret Westmacott and Stuart McCorrister at the Mass Spectrometry Core Facility at the JC Wilt Infectious Disease Research Centre.
This study was supported by The Canadian Institutes of Health Research (CIHR) (Author: ADB, TMI: 138658) and Public Health Agency of Canada (PHAC) (Author: ADB).
The authors declare that there is no conflict of interest regarding the publication of this paper. Preliminary findings were presented as a poster abstract at the 2020 World Congress in Ultrasound in Obstetrics and Gynecology.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
