- Academic Editors
Background: The traditional pathology of adenomyosis (AM) suggests that
this is a common benign uterine disease. Hysterectomy is the gold standard of
care and is viewed as a decisive treatment for AM; however, more conservative
treatment approaches are required to maintain fertility. Unfortunately, there are
few studies focusing on medical treatments for AM. The objective was to
investigate the effect of gonadotropin-releasing hormone agonist (GnRH-a)
combined with dienogest on serum human epididymis secretory protein 4 (HE4) and carbohydrate antigen 125 (CA125) levels in patients with AM and
adenomyoma. Methods: We addressed our objective using a prospective
cohort design. We selected 120 patients treated for AM and adenomyoma from
February 2019 to April 2021 in our hospital. The patients were divided
into a control group and a study group. The control group was treated with GnRH-a
alone, while the study group was treated with GnRH-a combined with dienogest. The
curative effect, dysmenorrhea score, dysmenorrhea grade, serum CA125 and HE4
levels, size of the uterine lesions, and incidence of adverse reactions were
compared between the two groups. Results: When comparing the two groups,
the study group consisted of 43 cases that were significantly effective, 12
effective cases, and five ineffective cases, thus, the effective rate was
91.67%. The control group displayed 23 significantly effective cases, 21
effective cases, and 16 ineffective cases, thus the effective rate for the
control group was 73.33%. The curative effect in the study group was higher than
in the control group, and the difference was statistically significant (p
Traditional adenomyosis (AM) pathology is indicated by the presence of ectopic endometrial glands and/or mesenchymal stroma, with growth at least 2.5 mm below the endometrial-myometrial interface (EMI) [1] within the endometrium. The development of such structures is due to a combination of factors along with adjacent smooth muscle proliferation, resulting in limited or diffuse hypertrophy of the subfloor myometrium. AM is a common benign gynaecological disease of the uterus with increased menstrual flow, prolonged menstruation, progressive dysmenorrhoea and secondary infertility as the principal clinical symptoms [2, 3]. Such complications result from in limited or diffuse hypertrophy of the myometrium and loss of normal myometrial structure [4, 5]. Although benign in nature, adenomyoma can cause complications such as increased menstrual flow, dysmenorrhoea, and infertility due to the invasion of endometrial glands, as well as the mesenchyme, into the myometrium to form diffuse or confined lesions. Anatomically, adenomyoma is characterised by ectopic growth of normal endometrial tissue in the myometrium and compensatory hypertrophy of the smooth muscle of the myometrium, resulting in an enlarged uterus. In addition, anaemia and even dysfunction of vital organs such as the heart, brain, and lungs have been attributed to adenomyoma due to the increased blood loss of the patient during menstruation. Additionally, lower abdominal cramps, frequent urination, constipation, or difficulty in urination due to pressure from an enlarged uterus [6, 7, 8] can occur. These complications can exert a serious impact on the physical and psychological health of women.
Human epididymis secretory protein 4 (HE4) is an emerging tumor marker that is highly expressed in ovarian epithelial cell carcinoma but not in normal ovarian tissue. Immunohistochemical results showed that HE4 was expressed in 100% of endometrial cancers, 93% of plasmacytic adenocarcinomas, and 5% of clear cell ovarian cancers [9]. As with another cancer marker, carbohydrate antigen 125 (CA125), serum HE4 levels are elevated in more than 80% of ovarian cancer patients. In addition to being used as a tumor marker to screen for malignancy, HE4 has implications for the prognosis of ovarian cancer patients and for monitoring the outcome of postoperative treatment. Available studies [10] have largely focused on the significance of HE4 in the diagnosis and differentiation of benign and malignant ovarian cancer, and some have mentioned uterine AM in the literature only as a control group for ovarian cancer studies. Moreover, a few studies have noted the importance of HE4 in the diagnosis of uterine AM and its differential diagnosis with uterine fibroids. However, the expression of HE4 in ovarian endometriosis stemming from ectopic endometrium has been studied more in recent years. For example, some investigators have demonstrated that the level of HE4 in serum from patients with uterine fibroids is not statistically significant when compared with normal controls. Thus, HE4 is potentially useful as a differentiating indicator between uterine AM and uterine fibroids [11, 12]. Other studies have shown that HE4 is highly expressed in all stages of endometrial cancer, with no significant differences between stages [13]. This appears true in early stage endometrial cancer as HE4 is a more sensitive serum marker than CA125. Therefore, we speculate that HE4 and CA125 can be used as indicators to assess the outcome of patients with AM and/or adenomyoma.
At present, the principal treatment options for adenomyoma are surgery and pharmacological therapies. Surgical treatment options include hysterectomy or focal resection, both of which have proven clinically effective. However, surgical treatments can be traumatic for patients as hysterectomy is not only represents a loss of fertility for the patient, but also affects the blood supply to the ovaries [14]. These outcomes make it difficult for some patients to accept. Pharmacological treatment includes the use of prostaglandin inhibitors, danazol, progesterone, gonadotropin-releasing hormone agonist (GnRH-a), and blood stopping. In clinical practice, conservative treatment of uterine adenomyoma is largely based on GnRH-a, which depletes the presynaptic receptors of hypothalamic-pituitary transmission and produces a negative feedback effect. Specifically, GnRH-a inhibits gonadotropin release from the pituitary gland causing a decrease in luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels. This lowers the release of ovarian hormones resulting in low estrogen levels and a “reversible drug depot” phenomenon [15]. GnRH-a also reduces the blood supply to the patient’s uterine adenomyoma, thus achieving the desired therapeutic outcome of relieving symptoms such as dysmenorrhoea and reducing lesion size [16]. However, due to side effects and the tendency to relapse after discontinuation, patients’ compliance is generally poor. Moreover, long-term use of GnRH-a may result in bone loss due to low estrogen status and/or calcium in the body, and patients are prone to menopause-like symptoms such as night sweats and mood swings [17].
Dienogest (DNG) is a synthetic progestin, and a component of the contraceptive progestin, has only recently been introduced for the treatment of sub-terminal AM. Dienogest reduces the patient’s estrogen levels by mediating the hypothalamic-pituitary-ovarian axis, creating an inhibition of the ovarian endocrine system and simultaneously blocking the synthesis process of estrogen metabolizing enzymes [18]. Dienogest also has an anti-inflammatory effect and inhibits scarring and blood vessel formation, thereby directly inhibiting the creation of ectopic lesions [19]. Dienogest has both the pharmacological characteristics of a natural progestin and a synthetic hormone and greatly increases the activity of the progestin. After 3 months of administration, dienogest effectively improves the associated pain symptoms and is better tolerated by the patient [20]. A large number of studies and clinical data show that dienogest tken at 2 mg/day is safe and effective in the treatment of endometriosis, but its effectiveness in the treatment of sub-terminal AM is still in initial trials [21, 22, 23]. Based on this, the present study investigated the effect of combined treatment of GnRH-a and dienogest on the serum levels of HE4 and CA125 in patients with AM and adenomyoma, as well as the treatment efficacy of this therapeutic combination.
One hundred and twenty (120) patients with AM and adenomyoma treated in our
hospital from February 2019 to April 2021 were selected for this study. The
patients were and evenly divided into a control and a study group. The
control group was treated with GnRH-a and the study group was treated with GnRH-a
combined with dienogest. In the control group, the age was 21–44 years and
average age was 32.56
Inclusion criteria: (1) Married non-menopausal patients aged between 30 and 50 who required conservative treatment and resolutely refused surgical treatment; (2) Clinical symptoms of dysmenorrhoea and increased menstrual flow; (3) Ultrasound indicated limited adenomyoma with a lesion diameter of 4–7 cm; (4) Patients had not received related prior treatments and had not taken related hormone drugs within a six month period; (5) Patients who had excluded endometrial and cervical lesions before treatment; (6) Patients who could communicate and cooperate fluently with the investigators.
Exclusion criteria: (1) Pregnant or lactating women; (2) Patients with cancer,
suspected malignant tumors or elevated tumor markers; (3) Patients with widely
dispersed AM and enlarged uterine volume; (4) Patients with a body mass index
(BMI)
The control group was treated with a 3.6 mg intramuscular injection of gonadotropin-releasing hormone antagonist (GnRH-an) and GnRH-a (Diphereline, Epson BioTechnology, Lavallois Perret, France), every 28 days for six consecutive cycles. The study group was treated with dienogest in combination with GnRH-a by taking 1 capsule/day of dienogest (Bayer, Leverkusen, Germany) at 28-day intervals for 6 weeks.
Dysmenorrhea was evaluated according to the visual analogue scale (VAS). The reduced degree of dysmenorrhea was more than two normal menstrual cycles in 80% of patients, complete remission was noted in 50% of patients, significant remission was observed in 20% of patients, slight remission was observed in 50% of patients, and an anti-menopausal effect was observed in 20% of patients. Per the difference in evaluation scores before and after treatment, the curative effect on menstrual volume was divided into 3 points, 2 or more points, 1 or more point, and 0 points corresponding to complete remission, significant remission, slight remission, and mild remission, respectively. Evaluation of patients with dysmenorrhea and menstruation scores that indicate complete or significant remission also displayed the return of a normally shaped uterus. Generally, treatment was judged to be effective when the lesion area was reduced by more than 70% and the reduced lesion area was judged to be significant between 30% and 70%. Evaluation scores showed that reduced uterine volume or a focus area of less than 30% was judged to be effective. The overall effective rate was quantified using the equation: Effective rate = (number of effective cases + number of effective cases)/total number of cases.
The menstrual volume score was recorded using a Pictorial Blood Loss Assessment
Chart (PBAC) [24, 25], and all patients used uniform sanitary napkins. The
scoring criteria were as follows: Blood-stained area
All patients included in the study had 3–5 mL of blood drawn the morning before treatment, and 1, 3, and 6 months after treatment. Blood draws avoided the menstrual cycle and were collected in a vacutainer tube with separation gel. HE4 and CA125 were measured using Elecsys HE4 and Elecsys CA125 kits from Roche, Basel, Switzerland. Electrochemiluminescence immunoassays were performed automatically and in strict accordance with the kit and instrument instructions.
Uterine lesion sizes were examined using transvaginal ultrasound. The changes in
focus size were observed before and after treatment, and the lesion volume,
length diameter (D1), left and right diameter (D2), and anterior and posterior
diameter (D3) were measured. The uterine lesion volume was calculated by the
formula: Vbelt = 0.5233
The incidence of adverse reactions in both groups was assessed by avoiding the menstrual cycle and included headache, breast discomfort, and mood changes. Headache refers to pain in the upper part of the skull, including the arch of the eyebrow, the upper part of the auricle, and the area above the line of the external occipital ridge. Breast discomfort primarily includes breast swelling and pain, nipple overflow, and nipple skin itching. Mood changes included emotions that cannot be calmed, such as irritability, and cannot be relieved for 2–3 days and are accompanied by a series of physiological changes.
All data generated or analysed during this study are included in this
manuscript. SPSS21.0 (IBM Corp., Armonk, NY, USA) statistical software was used
for statistical analysis. Prior to statistical analysis, measurement data were
tested for normal distribution and variance homogeneity. Repeated measurement
data were analysed by repeated measurement analysis of variance. A
t-test was used to compare the two groups, n (%) was used as an example
to represent the counting data, and the
In comparing the curative effect between the two experimental groups, the study
group had 43 significantly effective cases, 12 effective cases, and 5 ineffective
cases; thus, the effective rate of a combination of dienogest and GnRH-a was
91.67%. The control group (GnRH-a only) had 23 significantly effective cases, 21
effective cases, and 16 ineffective cases; thus the effective rate for GnRH-a was
73.33%. Overall, the curative effect in the study group was higher than in the
control group, and the difference was statistically significant (p
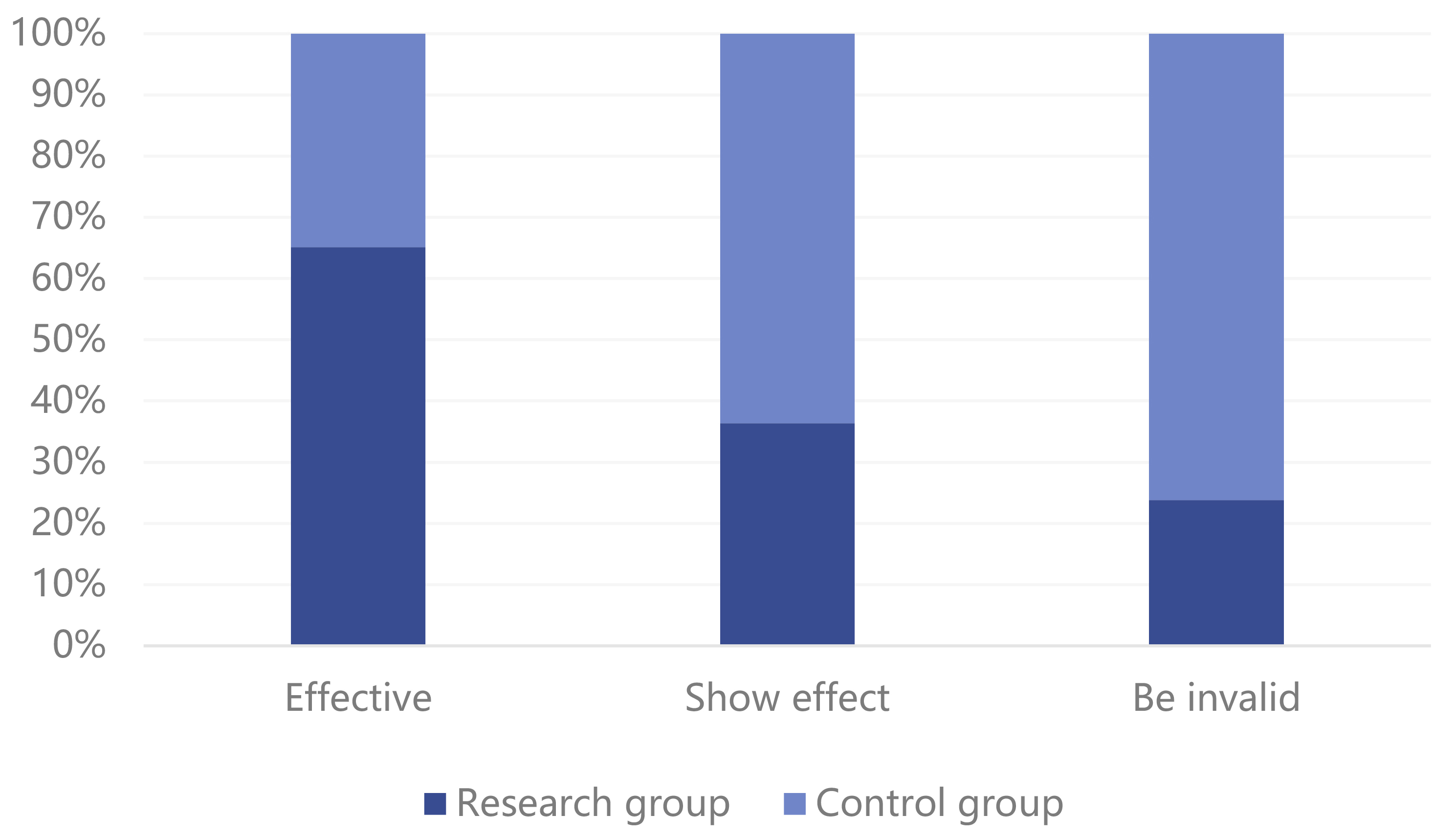 Fig. 1.
Fig. 1.Comparison of curative effect between two groups of patients.
The dysmenorrhea score showed no significant difference between the two groups
prior commencement of treatment (p
| Group | Case | Before treatment | One month after treatment | Three months after treatment | Six months after treatment | F | p |
| C group | 60 | 6.38 |
5.11 |
3.95 |
2.43 |
191.970 | |
| R group | 60 | 6.34 |
4.04 |
3.01 |
1.45 |
275.635 | |
| t | 0.120 | 21.654 | 16.770 | 16.765 | |||
| p |
C group, control group; R group, research group.
There were no significant differences noted in the levels of serum CA125 or HE4
between the two groups prior treatment. However, the levels of serum CA125 and
HE4 decreased following treatment. Of note, the levels of serum CA125 and HE4 in
the study group were lower than in the control group and the difference was
statistically significant (p
| Group | Case | Before treatment | One month after treatment | Three months after treatment | Six months after treatment | F | p |
| C group | 60 | 43.18 |
30.41 |
23.18 |
18.69 |
580.246 | |
| R group | 60 | 43.91 |
19.19 |
13.42 |
10.12 |
1271.104 | |
| t | 0.751 | 25.662 | 22.266 | 19.238 | |||
| p |
CA125, carbohydrate antigen 125.
| Group | Case | Before treatment | One month after treatment | Three months after treatment | Six months after treatment | F | p |
| C group | 60 | 7.83 |
4.59 |
3.85 |
3.06 |
135.133 | |
| R group | 60 | 7.81 |
3.56 |
3.01 |
2.74 |
828.816 | |
| t | 0.075 | 3.565 | 18.207 | 7.456 | |||
| p |
HE4, human epididymis secretory protein 4.
| Group | Case | Before treatment | One month after treatment | Three months after treatment | Six months after treatment | F | p |
| C group | 60 | 93.95 |
75.53 |
69.95 |
56.43 |
127.536 | |
| R group | 60 | 93.53 |
70.04 |
63.01 |
48.45 |
137.672 | |
| t | 0.137 | 2.107 | 4.890 | 4.215 | |||
| p |
There was no significant difference measured in the size of uterine lesions in
patients enrolled in either group before treatment (p
There was no statistically significant (p
 Fig. 2.
Fig. 2.Comparison of the incidence of adverse reactions between the two groups.
Adenomyoma is the most common benign tumor occurring in female reproductive organs. It is a common tumor in women from 30–50 years of age with the age of onset becoming younger in recent years. The principal manifestations of adenomyoma are increased menstrual flow and secondary dysmenorrhoea, which not only affects the patient’s physical health, but also her psychological well-being. The pathogenesis of adenomyoma is not fully understood, but studies have found that factors such as multiple abortions, intrauterine device (IUD) insertion, and caesarean sections are causative factors for adenomyoma [26, 27]. In addition, some studies have shown that elevated estrogen levels are also a cause of adenomyoma [28, 29, 30]. Although adenomyoma is benign in nature, it can cause complications such as increased menstrual flow, dysmenorrhoea, and infertility each of which can seriously affect women’s physical and psychological health. Moreover, adenomyoma can endanger their lives due to aggressive growth and consequential alteration of myometrial structure and malignancy-like biological behavior.
Gonadotropin-releasing hormone (GnRH) is a decapeptide neurohormone produced by the hypothalamus and is a key neuroendocrine regulator of the hypothalamic-pituitary-gonadal axis which plays an important role in the regulation of the reproductive system. GnRH-a is a derivative obtained by substituting amino acids at positions 6 and 10 in the natural structure of GnRH [31, 32, 33]. GnRH-a competes with GnRH for GnRH receptors in the anterior pituitary gland which depletes the number of GnRH receptors. This subsequently reduces the stimulatory effect of GnRH on the pituitary gland and inhibits gonadotropin release [34]. In clinical studies, GnRH-a is widely used in the treatment of uterine fibroids, endometriosis, AM, precocious puberty, assisted reproduction techniques, and preservation of fertility in chemotherapy patients. GnRH-a drug therapy is somewhat reversible and the clinical symptoms of AM tend to recur after discontinuation of the drug. Moreover, as the dosage of this type of drug is high, it is expensive, and the side effects are more pronounced for patients with uterine AM, patients have relatively poor drug adherence rates [35, 36, 37].
At present, much effort has focused on the creation of synthetic progestins with the dual properties of 19-nortestosterone and progesterone derivatives such as dienogest. Similar results have been obtained in trials of subendometriosis, where the efficacy of dinopregnanol, taken at 2 mg daily for 24 weeks, was comparable to that of sertraline in relieving the associated symptoms of pelvic pain, discomfort during intercourse, and low back pain. However, dinopregnanol reduced several symptoms including diastolic duct syndrome, reduced density, genital atrophy, and painful intercourse as well as symptoms that require the addition of small amounts of estrogen, including atrophy and painful intercourse, and increased tolerance and prolonged tolerance [38].
When comparing the efficacy of the two groups, the effective rate of the study
group was 91.67% and the effective rate of the control group was 73.33%. When
comparing between groups, the efficacy of the study group was higher than that of
the control group (p
For larger adenomyomas, GnRH-a alone is not the best option [39, 40, 41, 42]. GnRH-a
combined with dienogest can reduce the blood supply to the patient’s adenomyoma
lesions by reducing the estrogen and blood supply to the adenomyoma lesions. The
incidence of adverse reactions in the two groups was examined and there was no
difference observed between the study group and the control group, and the data
difference was not statistically significant (p
CA125 is both a macromolecular glycoprotein and a membrane antigen. CA125 has
been found to be present on the cell surface of ectopic endometrial lesions, and
ectopic endometrium has a strong capacity to synthesize and secrete CA125 up to
four times higher than in normal endometrium [45, 46, 47]. HE4 is secreted by ovarian
cancer cells and has a molecular weight of approximately 12 kD compared to CA125
which has a molecular weight of 200,000 to 1 million kD. It has been hypothesized
that this is the reason why HE4 is secreted into the bloodstream earlier and more
readily than CA125 in ovarian cancer patients. Therefore HE4 is viewed as
detecting ovarian cancer at an earlier stage. Combined with the results of this
study, serum CA125 and HE4 levels in the study group were lower than those in the
control group (p
In conclusion, the use of GnRH-a in combination with dienogest has shown a significant reduction in serum CA125 and HE4 levels, menstrual flow, dysmenorrhoea and change in lesion size in patients with AM and adenomyoma compared to the group receiving GnRH-a alone. The combination treatment regimen resulted in a more effective reduction in clinical symptoms, reduction in lesion size, and improved treatment efficiency. We conclude that GnRH-a, when combined with dienogest, is a superior choice for women with larger adenomyoma lesions requiring conservative treatment.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
DGW and LMJ designed the research study. CLJ and MJS performed the research. LMJ and CLJ analyzed the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This study was approved by Jinhua Hospital Zhejiang University School of Medicine Institutional Review Board (No. 2018-336), and all patients signed informed consent.
We would like to express our gratitude to all those who helped us during the writing of this manuscript.
This research was funded by Jinhua Science and Technology Bureau Project (No: 2021-3-126).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
