- Academic Editor
Background: Conventional cervicovaginal Papanicolaou (Pap) stained smears are a common investigation in gynaecological practice for detecting cancerous and precancerous cervicovaginal lesions, as well as infections and inflammatory processes. Although Candidiasis is the most common fungal infection detected on Pap smears, cytopathologists sometimes also have to deal with the challenge of fungal contaminants. The aim of this study is to present and discuss the findings of two rare fungal organisms in the context of previous literature reports. Methods: Over a period of one year, 4496 smears were submitted to the cytopathology laboratory for analysis. These were sampled from women aged 16–72 years. Slides were processed using the conventional Papanicolaou stain (Pap stain) method. The current available literature was reviewed using relevant key words. Results: Of the 4496 smears examined, the most frequently detected fungal species was Candida species spp. (523 cases), followed by Gardnerella Vaginalis (450 cases) and Trichomonas Vaginalis (50 cases). Also identified were 20 cases with Actinomyces spp. and 18 cases with unusual contaminants belonging to Penicillium and Alternaria spp. A literature search found that five previous articles reported cervical cytology cases with Penicillium and Alternaria spp. Conclusions: Papanicolaou smears are useful for the detection of vaginal microorganisms. Usual pathogenic flora need to be distinguished from contaminants such as Penicillium and Alternaria spp., as observed in this and previous studies.
The Papanicolaou (Pap) stain is a cost-effective method developed by George Nicholas Papanicolaou for detecting the precursors of gynecologic cancer and for diagnosing specific infections. Infections detected by Pap tests can have a viral, bacterial, fungal or parasitic etiology. The most common organisms detected on cytologic preparations are Human Papilloma Virus, Herpes Simplex Virus, Actinomyces spp., Gardnerella and Trichomonas Vaginalis, with Candida spp. being the most frequent fungal infection detected by Pap smear [1, 2]. However, there are also rare findings of fungal infections with coccidioidomycosis, Blastomyces dermatitidis, Penicillium and Alternaria spp. in routine and conventional cervicovaginal Pap smears. In such cases it is crucial to differentiate between a true infection and contamination. The aim of the present study is to raise awareness amongst physicians of rare contaminants such as Penicillum and Alternaria spp. that can be identified on Pap smears.
We evaluated slides from 4496 cases submitted to our cytopathology laboratory over a one-year period (1 November 2021–30 October 2022), and also reviewed the current literature on this topic. Cervicovaginal smears were obtained from women aged 16–72 years, with a mean age of 48 years. The purpose of the study was to identify specific infections that may be encountered in Pap smears, and to conduct a differential diagnosis between true infection and contamination. All slides were processed in the same manner with the Papanicolaou conventional stain (Pap stain) method, and all samples were received by the same laboratory from different gynecologic practices and patients. The number of slides varied between different medical practices. We used a modified Pap stain method [1, 2] as follows: I, Mayer haematoxylin (2 minutes, Bio-Optica, Milano, Italy); II, tap water (10 dips); III, ethanol solution, 96% (2 minutes, Chimreactiv, Bucharest, Romania); IV, OG-6 (orange gelb, 2 minutes, Merk, Damstadt, Gemany); V, ethanol solution, 96% (2 minutes); VI, EA-50 (ethyl alcohol, 2 minutes); VII, ethanol solution, 96% (2 minutes); VIII, toluene solution (5 minutes, Lach-Ner, Neratovice, Czsech Republic); and IX, mounting of coverslips with entellan medium (Merck, Darmstadt, Germany). All solutions were purchased commercially and ready-to-use. The inclusion criteria: solutions that respect the specific concentrations required for the stain. The exclusion criteria: expiration date of less than 1 year from the date of purchase.
English-language literature published over the last 10 years and covering the diagnosis of fungus on Pap smears was searched by accessing the PubMed electronic database and other sources. The following key words were used in the search: “cervicovaginal Pap smears”, “Penicillium”, “Alternaria”, “fungus” and “contaminants”. A total of 60 studies were identified. Abstracts and articles not in the field of interest were excluded, together with those containing irrelevant topics or repetitive information. The final number of articles was 23. These were divided into papers that reported the latest information on contaminants (n = 18), and those that presented cases where Penicillium and Alternaria spp. were identified as contaminants in cervical cytology smears (n = 5). The algorithm used for article selection in this study is shown in Fig. 1.
 Fig. 1.
Fig. 1.Algorithm used for article selection. Pap, papanicolaou.
Of the 4496 cases investigated, 29.19% showed the common pathogens. Only 18 cases (0.4%) contained the fungal organisms Penicillium and Alternaria spp. that are considered to be contaminants (Table 1). The results obtained for these two contaminants are described in more detail below.
| Organism | Frequency | Percentage (%) |
| Candida spp. | 523 | 11.63 |
| Gardnerella vaginalis | 450 | 10 |
| Trichomonas vaginalis | 50 | 1.11 |
| Actinomyces spp. | 20 | 0.44 |
| Penicillium spp. | 10 | 0.22 |
| Alternaria spp. | 8 | 0.17 |
Of the 4496 samples submitted to the cytopathology laboratory, 18 showed features consistent with an uncommon fungal infection. A rare fungal organism was identified in 10 cases sampled by the same physician from healthy females aged 22–57 years (Figs. 2,3). These samples were processed in one batch and at the same time. A microorganism with hyphae that branch into brush-like structures was identified on these slides (Fig. 2). Some of the structures had blunt tips and some had spores arranged in a chain-like pattern (Fig. 3).
 Fig. 2.
Fig. 2.Hyphae branching into brush-like structures. Superficial and
intermediate squamous cells and neutrophils cells can be seen in the background
(cervical smear, 400
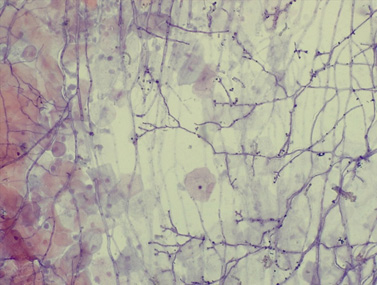 Fig. 3.
Fig. 3.Characteristics of the fungal organism Penicillium
spp. Fungal organism consistent with Penicillium spp. and showing
branching hyphae and blunt tips with spores arranged in a chain-like pattern.
Superficial and intermediate squamous cells can also be seen (cervical smear, 400
Following careful examination, it was concluded the fungal organisms were not in the same plane as the cells on the slides, and that few inflammatory cells were present in the background. The final cytology report noted the presence of a fungal organism with features consistent with Penicillium spp. and likely due to contamination. The referring physician was informed, and following investigation at their practice the source of contamination was identified as being the spatulas. In the months following these cases, no other fungal infection with Penicillium spp. was noted for the slides received from this physician.
We also found 8 cases with a brown fungal organism that showed morphological features consistent with conidia of Alternaria spp. (Fig. 4). These were found in patients aged 28–61 years.
 Fig. 4.
Fig. 4.Morphological characteristics of the fungal organism
Alternaria spp. Cluster of conidia with brown colour and a morphological aspect
consistent with Alternaria spp. (cervical smear, 200
All samples were received by the cytopathology laboratory during different times of the year and from different gynecologists. The smears were from isolated cases processed days or weeks apart, as well as samples that showed no Alternaria spp. fungus. Therefore, contamination from within the laboratory was ruled out. The cases with Alternaria spp. did not show significant inflammation on their smears and had no clinical complaints upon examination. Hence, the presence of this fungus was considered to be a contaminant.
Our results revealed a low incidence of infection with Actinomyces spp. (20/4496) and with Trichomonas Vaginalis (50/4496). The 20 cases with Actinomyces spp. showed filamentous bacteria in the form of dense basophilic balls within an inflammatory background. Intrauterine devices were present in 18/20 (90%) of these cases. The smears with Trichomonas vaginalis typically showed a pear-shaped organism with a small eccentric nucleus and an inflammatory background.
Pap-smeared cytology samples Papanicolau staining is important first of all for the early detection of cervical cancer, for the identification and analysis of the morphological aspects of the cells, but also for the detection of several infectious diseases with genital localization. Pap staining highlights the cytoplasm and nuclei of metabolically active cells such as superficial and intermediate squamous cells, parabasal cells, basal cells, endocervical cells, endometrial cells, inflammatory cells and microorganism (e.g., candida species, trichomonas or haemophilus vaginalis). The Pap stain method generally uses three main solutions: the hematoxylin stain for nuclei, and the OG-6 and EA-50 stains for cytoplasm. Additionally, water, 95% ethanol, xylene/toluene and mounting substances are used to achieve the final staining result [3, 4]. Since the initial publication of the Pap smear method, it has undergone several modifications and different laboratories now use modified protocols in order to obtain the best results.
Pap smear analysis uses non-invasive techniques to obtain samples. Moreover, the interpretation of results is affordable and fast, with accurate identification of microorganism and the detection of abnormalities that could indicate a risk of developing cervical cancer. Importantly, annual patient check-ups can be used to obtain smears on a regular basis. Furthermore, there is increasing interest in the use of smears to diagnose intrapartum and peripartum infections. Thus, the current evaluation of pregnancy involves screening for infectious diseases with a conventional Pap smear sample. Two types of immunity have been described during pregnancy. The type 1 immune response offers protection against fungal organisms like Candida, Coccidioides, Cryptococcus and Aspergillus. The type 2 immune response has been associated with increased susceptibility to viruses and also to pathogens such as Mycobacterium tuberculosis. Pap smears are considered useful for identifying infections with human papilloma virus (HPV), cytomegalovirus, and sexually transmitted infections with Gardnerella vaginalis (GV) and Trichomonas vaginalis (TV) in pregnant patients. HPV can cause respiratory papillomatosis in infants and children and interfere with the delivery. Cytomegalovirus is congenitally transmitted in about 40% of cases and is associated with spontaneous abortion and placental infection. GV and TV infections are also associated with an increased risk of preterm birth [5]. The diagnosis of a true infection and its differentiation from a contamination has major implications for the subsequent management of patients.
Contaminants and artifacts present important challenges when assessing Pap smears, and a lack of knowledge and experience can lead to them being confused with other structures. Contaminants are particles, organisms or other phenomena that alter the aspect of a Pap smear by obstructing or preventing the proper analysis of a cytological sample [1]. These may have an intrinsic or extrinsic origin and are considered to represent potential diagnostic pitfalls in cytodiagnosis. Contaminants can be in the form of bacterial flora and fungi from non-sterile instruments, dust, pollen grains, cotton swabs, patient cells, hair, commercial tampons, cotton and synthetic fibre [6]. Artifacts reported in Pap smears can arise from air-drying, air trapped under the cover slip, brush artifacts and lubricating gels [7].
English-language literature was investigated by assessing the PubMed electronic database and other sources using relevant key words (Fig. 1).
Infections that are rarely identified by the Pap test include molluscum contagiosum, cytomegalovirus, bacterial-tuberculosis, donovanosis, syphilis, malakoplakia, schistosomiasis, Tinea, Enterobius vermicularis and Entamoeba histolytica [8, 9]. Contamination of pap smears with Penicillium and Alternaria spp. fungal organisms is rarely reported in the current literature. We found 7 cases where Penicillium spp. was noted in the cervical cytology report. Brimo et al. [1] described 5 such cases and Donta et al. [10] one case. Martínez-Girón and Fernández-García identified fungal spores in a conventional Pap smear and reported these as being consistent with Aspergillus/Penicillium spp. spores. Cases of Penicillium spp. that caused lung and ophthalmologic infections have been described in immunocompromised hosts [1, 10, 11]. Our search of the literature database found only two articles that described Alternaria spp. isolated from the uterine cervix. One reported a single case, while the other analysed different types of contaminants and concluded that Alternaria spp. was likely to be one of them [12, 13]. Studies that have reported cases of contamination with Penicillium and Alternaria spp. are summarised in Table 2 (Ref. [1, 10, 11, 12]).
| Reference (author, year) | Type of study | Type of contaminant | No. of cases |
| Brimo F et al. (2009) [1] | Case report and literature review | Penicillium spp. | 5 |
| Donta B et al. (2010) [10] | Case report | Penicillium spp. | 1 |
| Martínez-Girón R et al. (2009) [11] | Case report | Penicillium spp. | 1 |
| Erşahin C et al. (2005) [12] | Case report | Alternaria spp. | 1 |
We identified 18 cases of contamination in the present study of patients with otherwise unremarkable examination findings. Ten of these had features that were characteristic of Penicillium and 8 for Alternaria spp. We classified these two fungal organisms as contaminants based on five criteria: (1) Penicillium spp. was identified in just one batch of samples provided by the same physician; (2) follow-up revealed there were no other slides with Penicillium spp.; (3) the possibility of contamination from within the cytology laboratory was excluded because no other smears in the batch had Penicillium; (4) the hyphae and spores of Penicillium spp. were located in a different plane to the cervical cells, suggesting they were deposited on the slides at different times; and (5) the smears with Alternaria were found randomly on different slides obtained from different practices.
Several other features also indicated the presence of contamination and are described here. The cases were asymptomatic patients who presented for a routine medical check-up and who had an uneventful clinical follow-up. A repeat Pap smear was performed two weeks later for each patient. No contaminants were found on the repeat smears, indicating the initial findings were due to contamination.
For these diagnostics, Penicillium spp. had to be differentiated from Paecilomyces and Aspergillus spp., and Alternaria from Ulocladium and Fusarium.
Penicillium is found in the soil, air and decaying organic matter and displays hyphae with narrow conidiophores stipe [1, 14]. These fungal species consist of branched networks of hyphae with cells separated by septum. Conidiophores with spherical conidia develop at the end of these branches. The conidiophores have a brush-like appearance due to branching metulae and phialide which bears the conidia. The phialide have tapered ends from which the conidia/fruiting bodies arise in the form of chains [10, 15]. Philiades from the Paecilomyces species have a broader base and the clusters are arranged more loosely than Penicillium [1].
Aspergillus can resemble a collection of intermeshed fungal hyphae and
spores. The hyphae are 4–6
Alternaria is a fungal genus divided into 24 sections based on molecular and morphological data. Because Alternaria has variable morphological characteristics, molecular analysis is needed to diagnose this specific genus. Its main morphological feature consists of brown pluricellular conidia with longitudinal transverse septa. The conidia have a broad base and elongated tip. Alternaria has a world-wide distribution, with species being identified in the soil, air decaying organisms and plant pathogens, as well as on normal human and animal skin and conjunctiva. It has also been isolated from patients with asthma, pneumonitis, sinusitis, rhinitis, ocular infections, onychomycosis, cutaneous and subcutaneous infections, and from intra-abdominal and para-umbilical regions [18, 19]. Fungus belonging to Alternaria spp. can cause opportunistic infections in humans, mainly with cutaneous clinical manifestations. Alternaria spp. was also identified on contact lenses and in emollient cream [19, 20, 21].
Ulocladium botrytis is a newly described human pathogen that causes allergic fungal sinusitis. However, this fungus favours infections that are localised in the respiratory tract, and the conidia has a particular aspect. Muriform verrucose conidia with bent conidiophores, round endings and an oblique septation of the conidium have been described for Ulocladium [22].
Fusarium is a soil saprophyte isolated from contact lenses and catheters and also reported in women with cervical erosion. Fusarium species show macroconidia with a transverse septa formation. An exclusion diagnosis involving molecular methods and cultures is needed to distinguish Fusarium from other species [23].
Our study had the following limitations: the study design was retrospective, only a small number of cases had contaminants, the data was derived from just one laboratory, the method of analysis was microscopic assessment of Pap smears, and only a small number of relevant literature articles have been published so far.
The Pap screening test is useful for detecting infections and inflammatory processes. It is a safe test that can be performed even in the early stages of pregnancy. Differential diagnosis between a true infection and contaminants is crucial and saves patients from unnecessary antifungal therapy and additional laboratory tests.
So far, there are few published studies on Pap smear contaminants. Following analysis of the available literature, the present study is to our knowledge the largest to date on these unusual contaminants and the first based on the analysis of Romanian patients.
All data generated or analyzed during this study are included in this published article.
ACF and DCL designed this research study. ACF and CVG performed the research. LAG provided help and advice with analysis of the data. ACF and CVG co-wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity.
The patients were previously informed and gave their consent for the protocol and interventions used in this study. The study was retrospective, requested by a private cytology laboratory with the purpose of identifying pathogens, infections and contaminants on Pap smears and rising awareness. All the samples and clinical data were provided by the laboratory, all the personal data and cytology laboratory data are protected data. In relation of forming an ethics committee this study falls in the category of “Not applicable”.
We are grateful to all of those who helped us during the writing of this manuscript, as well as the peer reviewers for their helpful opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
