- Academic Editor
Objective: Ligustrazine is an amide alkaloid, with the active substance being Chuanxiong. Also known as tetramethylpyrazine (TMP), ligustrazine has various pharmacological effects and has been used to treat a variety of diseases. Many studies have demonstrated a role for ligustrazine in the treatment of several obstetrical and gynecological diseases. However, most results on the efficacy of ligustrazine have been obtained from basic laboratory experiments, with few clinical studies having validated these results. Mechanisms: In this narrative review we analyze the available literature summarizing the role and mechanism of ligustrazine in the treatment of obstetrical and gynecological diseases. Findings in Brief: TMP shows good results for the treatment of endometriosis, preeclampsia, ovarian cancer, as well as other obstetrical and gynecological diseases through its regulation of cell proliferation, invasion and metastasis, inflammation, immune response, apoptosis, autophagy, angiogenesis, endothelial protection, and fibrogenesis. Conclusions: TMP is therefore a promising drug with great research potential. Of note, ligustrazine is a complementary or alternative medicine and not the primary treatment. And most studies to date are laboratory-based experiments with low evidence levels. More in-depth research is needed to determine the pharmacological effects of ligustrazine in the clinic.
Chuanxiong is a traditional Chinese herbal medicine first reported in Shennong’s Classic of Material Medica [1]. Many common Chinese patent medicines and prescriptions, including ligustrazine injection, ligustrazine phosphate tablets, Jiawei Foshou San (JFS), Xuefu Zhuyu decoction, and Si Wu decoction, are made from or contain chuanxiong.
Ligustrazine, also known as tetramethylpyrazine (TMP), is an amide alkaloid that is isolated and purified from chuanxiong and constitutes the active substance [2]. TMP has various pharmacological properties, including analgesia, anti-inflammatory, anti-tumor, anti-oxidative, anti-apoptotic, anti-angiogenic, endothelial protectant, blood activator, and stasis remover [3, 4, 5]. It is safe to use and has minimal adverse effects [6]. Ligustrazine has recently been used for the treatment of various diseases with good therapeutic results. The research progress to date on the role and mechanism of ligustrazine in obstetrical and gynecological diseases is summarized in this review.
Endometriosis (EMS) is a common disease that manifests as active endometrial tissue growing outside the uterus. Approximately 6–10% of women of childbearing age and 25–50% of infertile women experience EMS; 30–50% of women with EMS are infertile [7, 8]. Although EMS is a benign gynecological disease, it has malignant properties characterized by invasion, metastasis, angiogenesis and recurrence. The pathogenesis of EMS remains unclear. Previous studies have shown that cell invasion, apoptosis, angiogenesis, and immune and inflammatory response may be related to the development of EMS [9, 10]. EMS treatment primarily involves surgery and medication. All currently used hormonal therapies are effective for pain reduction. However, EMS tends to relapse following the discontinuation of treatment [11]. Although surgical excision of the lesions reduces EMS-associated pain, the recurrence rate is 10–15% at 1 year after surgery, and as high as 40–50% at 5 years [12]. Therefore, the identification of safer and more effective treatments for EMS is a clinical and research priority.
Ligustrazine is an active ingredient of the Chinese herbal formula and can inhibit the development and progression of EMS. It is therefore an acceptable compound to use for the treatment of EMS. The research progress to date regarding ligustrazine on EMS and the possible mechanism of action are summarized in Fig. 1.
 Fig. 1.
Fig. 1.Pharmacological effects of ligustrazine on EMS.
Ligustrazine ameliorates EMS via multiple pathways, including
wnt/
According to the theory of retrograde menstruation, invasion and metastasis of the ectopic endometrium are important steps in the development of EMS [13]. Matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs) are present in specific ratios to maintain stability of the extracellular matrix (ECM). An imbalance between MMPs and TIMPS promotes invasion of the endometrial tissue through ECM degradation and EMS development [14]. The expression of MMP-2, MMP-3, and MMP-9 in the ectopic endometrial tissue of patients with EMS are significantly increased compared to normal tissue [14]. Tan et al. [15] reported that MMP-9 and MMP-2 expression were positively correlated with the volume of ectopic endometrial tissue, and that ligustrazine inhibits MMP-2 and MMP-9 expression in the ectopic endometrium both in vivo and in vitro. Ligustrazine also upregulates the expression of TIMP-1, thereby inhibiting invasion and metastasis in EMS.
Epithelial-mesenchymal transition (EMT) is a cellular program whereby cells evolve from an epithelial to an mesenchymal phenotype and subsequently gain the ability to invade and metastasize [16]. EMT is an important pathological factor in EMS. Zhang et al. [17] showed that expression of the epithelial marker E-cadherin in an EMS model system was reduced, whereas expression of the mesenchymal biomarkers N-cadherin, Snail, Slug, vimentin, ZEB1 and Twist was increased. Treatment with ligustrazine inhibits ectopic lesions, while E-cadherin expression in EMS is markedly increased. In constrast, the expression levels of N-cadherin, Vimentin, N-cadherin, Snail, Slug, vimentin, ZEB1 and Twist are reduced, thereby inhibiting invasion and metastasis.
Estrogen plays a key role in the development of EMS [18]. High concentrations of
estradiol and upregulation of the estrogen receptors (ER)
Estrogen is produced in the ectopic lesions of EMS through a positive feedback
loop involving cyclooxygenase-2 (COX-2), prostaglandin E2 (PGE2), aromatase P450,
and estradiol (E2). Interleukin 1
An abnormal peritoneal microenvironment is an important factor for the induction
of EMS, with macrophages playing a key role in maintaining the normal peritoneal
environment [23]. Nuclear factor-
The effect of ligustrazine on the expression of EMS inflammatory factors involves other pathways. Chemokine receptor 5 (CCR5) levels in the peritoneal fluid and serum of patients with EMS are significantly higher than that in healthy women, and CCR5 levels in the peritoneal fluid are positively correlated with disease severity. CCR5 and its ligands can drive EMS progression by inducing the accumulation of myeloid-derived suppressor cells [25, 26]. Ligustrazine has an inhibitory effect on the expression of chemokine ligand 5 (CCL5) and its receptor, CCR5. This may halt any further local immune inflammation, thus affecting the development of EMS [27].
Apoptosis is a form of programmed cell death that facilitates homeostasis. Mutual coordination between the proliferation and apoptosis of endometrial cells leads to periodic proliferation and shedding of the endometrium. The pathogenesis of EMS may be related to an imbalance between the proliferation and apoptosis of ectopic endometrial cells [28]. Activation of apoptosis by ligustrazine in ectopic endometrial tissue may occur through the Bcl-2 apoptosis signaling pathway [29]. Wei et al. [22] reported that ligustrazine significantly inhibits the growth of endometriotic cells. Simultaneously, ligustrazine decreases expression of the anti-apoptotic factor Bcl-2, increases expression of the pro-apoptotic factors Bax and Bad, and increases the expression of caspase-3 and caspase-9, thereby activating the apoptosis of ectopic endometrial cells.
Similar to a malignant tumor, EMS involves excessive angiogenesis. Vascular endothelial growth factor (VEGF) is the most important cytokine that promotes angiogenesis in ectopic endometrial tissue [30]. Ligustrazine has an anti-tumor-like angiogenesis effect and can inhibit the expression of VEGF. Wang et al. [31] administered Jiawei Foshou San (JFS) intragastrically to three groups of EMS rats (50, 100, and 150 mg/kg doses). JFS is a new formulation of ligustrazine, ferulic acid and tetrahydropalmatine. All rats were treated daily for four consecutive weeks and analyzed by immunohistochemical staining and western blotting for microvessel density (MVD) and for VEGF and endostatin expression in the ectopic endometrium.The results showed that JFS inhibited MVD and VEGF expression in a dose-dependent manner,while promoting endostatin expression. Thus, JFS can hinder the progression of EMS by inhibiting angiogenesis.
Increasing evidence suggests that EMS involves repeated tissue injury and repair, with platelets playing an important role in EMS progression [32]. Ligustrazine can inhibit platelet aggregation, thereby reducing fibrosis [4]. Huang et al. [33] also showed that ligustrazine can slow the progression and fibrogenesis of EMS by inhibiting platelet activation, platelet-induced EMT, fibroblast-myofibroblast transdifferentiation (FMT), cellular contractility, and collagen production, and reduce pain.
In summary, ligustrazine can inhibit the development and progression of EMS through the various mechanisms described above. Therefore, ligustrazine is a potential therapeutic agent for the treatment of EMS and its symptoms. Although some studies have reported various pharmacological effects from ligustrazine for EMS, these were limited to in vitro and in vivo experiments. Clinical trials of ligustrazine for EMS are lacking, and rigorous clinical studies are needed to verify its efficacy and safety. At present, most studies have focused on the effect of ligustrazine on the development and progression of EMS. Few studies have investigated whether ligustrazine can improve EMS-related infertility, with the related mechanisms still to be explored. Future research should be directed towards the use of ligustrazine to reduce side-effects of Western hormone therapies, and towards reducing the incidence of recurrent EMS.
Preeclampsia (PE) is a common pregnancy-related complication that can lead to many serious maternal and fetal conditions. The pathogenesis of PE is complex and may be related to placental ischemia and hypoxia, immune factors, endothelial dysfunction,and genetic factors. The exact pathogenesis of this disease has yet to be fully elucidated.
Traditional Chinese medicines such as TMP have been widely investigated in China for the treatment of PE. Several studies have shown that TMP inhibits the development of PE. Research progress to date on the effects and mechanisms of TMP on PE is summarized in Fig. 2.
 Fig. 2.
Fig. 2.Pharmacological effects of ligustrazine on preeclampsia. Ligustrazine has anti-preeclampsia effects through the regulation of trophoblast function, inhibition of ER stress, improved endothelial function, dilation of blood vessels, and improve microcirculation. Abbreviations: IGF, insulin-like growth factor; sFlt-1, soluble Fms-like tyrosine kinase 1; AGES, advanced glycosylation end products; RAGE, receptor of AGEs; GRP78, Glucose regulating protein 78; CHOP, human endoplasmic stress-related proteins; PGI2, prostacyclin I2; TXA2, thromboxane A2; VEGF, vascular endothelial growth factor; eIF2α, α subunit of eukaryotic initiation factor 2; p-eIF, phosphorylated eIF2α.
Trophoblasts are key regulators of placental development, with trophoblast dysfunction being associated with various pregnancy-related complications, including PE. MicroRNAs (miRNAs) are single-stranded, non-coding small molecules that negatively regulate gene expression and play an important role in a variety of diseases [34, 35]. Certain miRNAs are abnormally expressed in preeclamptic placental tissue and play key roles in regulating trophoblast function, suggesting they may be potentially useful therapeutic biomarkers for PE [36, 37].
Yuan et al. [38] found that miR-16-5p targets the IGF-2 gene and can downregulate its expression in JEG2 cells, thereby enhancing autophagy and reducing cell viability and migration. Treatment with ligustrazine inhibited the expression of miR-16-5p in JEG3 cells, which in turn inhibits autophagy and enhances cell viability and migration.
Endoplasmic reticulum (ER) stress plays a key role in PE pathogenesis [39].
Moreover, increasing evidence suggests the therapeutic effect of TMP may be
related to the anti-ER stress functions [40]. Treatment of PE rats with TMP
inhibited the expression of GRP78 and CHOP and reduced the ratio of
p-eIF2
Endothelial dysfunction is one of the mechanisms that underlies PE development. It leads to an imbalance of procoagulant and anticoagulant factors, resulting in a state of hypercoagulation and a series of reactions including coagulation system disorders [42]. Endothelial dysfunction also induces the intravascular inflammatory response [43]. Therefore, protection of endothelial cells is of great importance. Chen et al. [44] showed that salvia and ligustrazine hydrochloride injection improved clinical symptoms and pregnancy outcomes of women with PE. Possible mechanisms include regulation of the AGEs-RAGE signaling pathway, reduced expression of downstream inflammatory growth factors, weakening of the inflammatory response, and oxidative damage, thereby improving endothelial function.
VEGF plays an important role in normal vessel growth and in maintaining endothelial function. Soluble Fms-like tyrosine kinase 1 (sFlt-1) produced in the placenta increases the clearance of VFGF in PE, leading to widespread endothelial dysfunction [45]. Liu et al. [46] found that ligustrazine treatment increased the level of VEGF expression and decreases sFlt-1 expression in patients with PE, thus improving vascular endothelial function and pregnancy outcomes.
Systemic arteriolar spasm, hemoconcentration, and hypovolemia are the main pathophysiological changes associated with hypertensive disorders of pregnancy. Wang et al. [47] reported that ligustrazine and salvia injection have a significant therapeutic effect on gestational hypertension by inhibiting fibrinolysis and enhancing the microcirculation. Thromboxane A2 (TXA2)/prostacyclin I2 (PGI2) ratio imbalance and hemorheological changes play a role in the pathogenesis of PE [48]. Ligustrazine injection can increase the synthesis and secretion of PGI2 by vascular endothelial cells, regulate the balance of TXA2/PGI2, and treat hypertensive disorders of pregnancy by dilating blood vessels, reducing peripheral resistance, and increasing blood perfusion [49].
In summary, the pathogenesis of PE is complex. TMP affects the development and progression of PE by regulating trophoblast function, reducing ER stress, protecting endothelial function, and improving the microcirculation, thereby offering a possible treatment option for PE. However, the potential mechanisms by which TMP regulates PE warrants further investigation.
Ovarian cancer (OC) is one of the three major malignant tumors of the female reproductive system. The lack of early clinical symptoms and efficient detection methods, together with rapid disease progression, result in most OC patients being diagnosed with an advanced stage. In addition, the pathogenesis of OC is still unclear and there is currently no effective therapy, resulting in poor prognosis. Therefore, it is important to develop new and more effective therapeutic strategies for OC. Similar to other natural products, ligustrazine has been shown to exert anti-tumor effects. This article reviews recent research progress on the mechanism of action of ligustrazine in the treatment of OC, thus providing a reference for subsequent research and clinical application (Fig. 3).
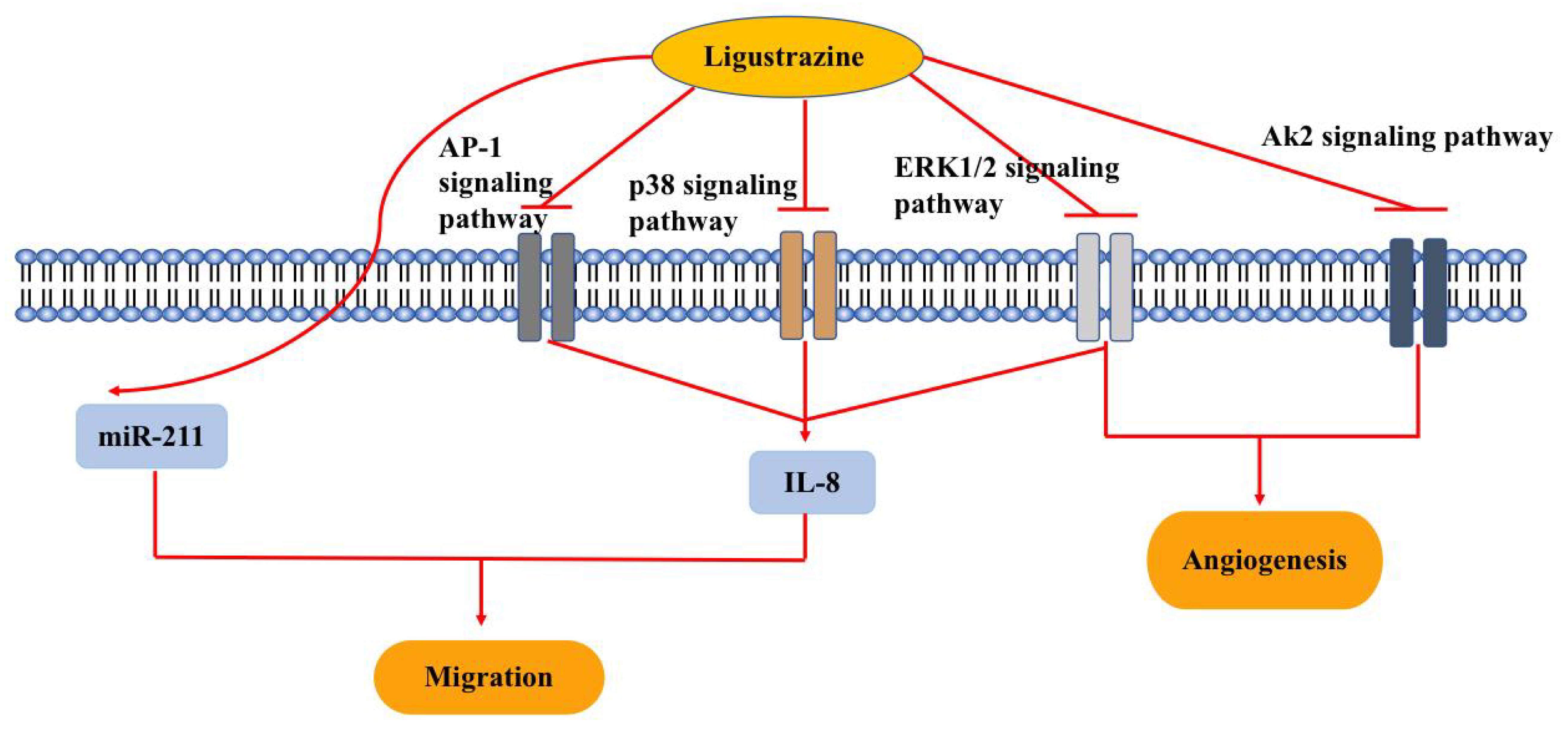 Fig. 3.
Fig. 3.Pharmacological effects of ligustrazine on ovarian cancer (OC). Ligustrazine reverses chemotherapy resistance, inhibits cell migration and angiogenesis through multiple signaling pathways, including Ak2, ERK1/2, p38 and AP1. Abbreviations: ERK, extracellular regulated protein kinases; AP, activator protein-1; AK, adenylate kinase; miR, microRNA; IL, interleukin.
Dysregulation of microRNA is a contributoty cause in several cancers types. Over the past decades, the role of miRNAs various cancer types has been studied extensively, with some miRNAs having been identified as potential therapeutic targets for OC treatment [50, 51]. Zhang et al. [52] used an in vitro model of OC to show ligustrazine inhibited the proliferation, migration, invasion, and EMT of the human OC SKOV3 cells by upregulating the expression of miR-211 in these cells. Thus, ligustrazine may exert its antitumor activity by increasing the expression of miR-211.
An association between inflammation and cancer is widely recognized. Interleukin-8 (IL-8) is a pro-inflammatory cytokine that is overexpressed in many malignant tumors. Anti-IL-8 antibodies have been shown to reduce tumor growth in a mouse cancer model. Merritt et al. [53] found that increased IL-8 expression was associated with poor clinical outcomes in OC and that IL-8 gene silencing could inhibit tumor growth, suggesting IL-8 may be a potential therapeutic target for OC. Yin et al. [54] confirmed that ligustrazine can reduce IL-8 expression through the ERK1/2, p38 and AP-1 signaling pathways, thus inhibiting the invasion and migration of human SKOV3 cells. Another study showed that ligustrazine inhibits the migration of SKOV3 cells induced by IL-8, which may be related to changes in E-cadherin and MMP-9 protein expression [55]. Following inhibition of IL-8 expression by ligustrazine, the expression of MMP-9 decreased and the expression of E-cadherin increased, thereby inhibiting the invasion and metastasis of human SKOV3 OC cells.
Angiogenesis is a key process in the proliferation and viability of tumor cells. Therefore, inhibition of angiogenesis is an important goal of tumor-suppressive therapy [56]. Zou et al. [57] found that TMP combined with paclitaxel (PTX) blocks angiogenesis by inhibiting the ERK1/2 and Akt pathways and by promoting tumor cell apoptosis, thereby inhibiting the progression of OC. TMP also enhanced the anti-tumor effect of PTX and reduced its toxicity.
Resistance to chemotherapeutic drugs is one of the main factors that reduces their efficacy against OC. Enhancing the sensitivity of chemotherapeutic drugs and reversing chemotherapy resistance are important research goals in tumor treatment. OC cells have a unique microenvironment, with numerous adipocytes in the peritoneal omentum playing an important role in the growth, metastasis, and drug resistance of ovarian tumor cells [58, 59]. Adipocytes can increase the expression of drug-resistant proteins in OC SKOV3 cells, thereby increasing their proliferation. Ligustrazine may also reduce the expression of drug-resistant proteins in tumor cells, thus reversing drug resistance [60].
In summary, ligustrazine is a traditional Chinese medicine extract that exerts anticancer and chemosensitizing effects through a variety of complex mechanisms. Compared to traditional chemotherapeutic drugs and chemosensitizers, ligustrazine has multiple sources, lower cost, better safety, and good prospects for application. It is worth noting however that most studies to date are laboratory-based experiments with low evidence levels. More in-depth research is needed to determine the anti-tumor effects of ligustrazine in humans.
Ligustrazine has multiple targets and pathways, and plays a key role in several obstetrical and gynecological diseases. Of note, ligustrazine is a complementary or alternative medicine and not the primary treatment. Furthermore, the majority of data on the efficacy of ligustrazine has been obtained from basic laboratory experiments, and few clinical studies have validated these results. Obtaining additional experimental results that could be used to guide clinical application is time consuming, requiring further work in this area. However, it is worth affirming that ligustrazine is a potential drug with likely beneficial applications in the field of obstetrics and gynecology.
YH, JC and HC designed the study. YH, AW and JC contributed to data acquisition and analysis. YH and AW drafted the manuscript. All authors have reviewed and edited the manuscript. All authors have contributed to the article and have carefully read and approved the final manuscript.
Not applicable.
Not applicable.
This research was supported by SiChuan Provincia Natural Science Foundation of China (Genaral Program) (2023NSFSC0738).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
