- Academic Editor
Background:
To explore the effectiveness of standardized first-trimester ultrasound screening
(FTS) in detecting fetal structural abnormalities in a non-selective population.
Methods: A retrospective study was performed on 7523 fetuses (6376
single and 569 twin pregnancies) who underwent FTS between 11 and 13
Since the incorporation of nuchal translucency (NT) in first-trimester ultrasound screening (FTS) for Down’s syndrome [1], it has become an indispensable tool for screening for fetal aneuploidy in conjunction with maternal serology [2, 3, 4]. Although cell-free DNA testing has minimized the role of NT in first-trimester aneuploidy detection, the advancement of ultrasound technology and knowledge of fetal anatomy has enabled early pregnancy screening for fetal structural abnormalities. As more than 80% of fetal structural abnormalities manifest prior to the 12th week of gestation [5], careful examination of fetal structures during the first trimester could facilitate early detection of fetal structural anomalies. Despite the widespread use of FTS for detecting fetal structural abnormalities, there is no universally accepted standard for evaluating its performance. Differences in study populations, screening protocols, and criteria for inclusion of malformations have resulted in varying results being reported. To shed light on this issue, we conducted a study using a standardized scanning protocol in a non-selected Chinese population, with the goal of further exploring the role of FTS in detecting fetal structural abnormalities.
This retrospective study analyzed data from women who underwent routine NT scan at the Affiliated Suzhou Hospital of Nanjing Medical University, China, between September 2017 and July 2021. The study was registered at https://www.chictr.org.cn (registration number ChiCTR-SOC-17010976). The study included a total of 7523 fetuses, comprising 6376 single pregnancies and 569 twin pregnancies. The study population had an average age of 29 years (interquartile range (IQR) 27–31) and the fetuses had a crown-rump length (CRL) of 45–84 mm, with a mean CRL of 66 mm (IQR 62–71). Prior to the examination, all study patients provided informed consent.
In this study, a Philips Affiniti 70 color ultrasound machine was utilized, equipped with a convex probe of C9-2 (frequency 2–9 MHz). All examinations were conducted transabdominally by 5 ultrasound physicians who were accredited by the Fetal Medicine Foundation (FMF) for FTS.
All ultrasound scans were conducted in a transabdominal manner, adhering to the FMF standard for fetal CRL and NT measurements, as well as Doppler evaluation of the blood flow across the ductus venous and tricuspid valve [6]. Additionally, we followed a more detailed anatomy scanning protocol based on the guidelines of the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) [7]:
(1) Transverse section of the fetal head to examine the skull, cerebral falx, lateral ventricle, and choroid plexus.
(2) Mid-sagittal section of the fetal head to observe the fetal face (nasal bone, maxilla, mandible, and chin) and intracranial structures (thalamus, midbrain, brainstem, fourth ventricle, and cisterna magna).
(3) Transverse section of the fetal eyes and retronasal triangle plane (RNT) to analyze the fetal eyes, palate, and upper lip.
(4) Two-dimensional and color-flow images of the 4-chamber cardiac view, the left ventricular outflow tract (LVOT) view, and the three-vessel-tracheal (3VT) view to examine the lungs, the fetal heart chambers, and great vessels.
(5) Parasagittal section of the upper chest to inspect the bilateral integrity of the diaphragm.
(6) Abdominal transverse section to assess fetal stomach and abdominal wall integrity and both cross and coronal sections of the lower abdomen to observe the fetal kidneys.
(7) Transverse section of the bladder to observe the fetal bladder and both umbilical arteries.
(8) Three-segment section of the upper and lower limbs to observe fetal limbs.
(9) Sagittal and coronal views of the spine to observe the fetal spine.
(10) If the ultrasound image was not satisfactory, a repeat examination at intervals of 20–30 minutes was performed, and the total scanning time was limited to half an hour in normal fetuses and one hour in abnormal fetuses. If necessary, further genetic testing was suggested. All acquired ultrasound images were stored in an ultrasound database.
Data on pregnancy outcomes in this study were obtained from the Jiangsu Maternal and Child Health Information System as well as by telephone surveys. Autopsies were performed on aborted fetuses. Severe structural malformations found during the first trimester, such as major cardiac malformations, were assessed by a specialized echocardiography physician, and then reviewed 2 weeks later to confirm the diagnosis. For ongoing pregnancies, results from later ultrasound examinations were documented, and the final pregnancy outcomes were monitored. Pediatricians conducted postnatal check-ups for all newborns, both immediately after birth and 42 days postdelivery.
Fetal findings considered to be structural abnormalities (regardless of chromosomal abnormalities):
(1) Fatal malformations, i.e., anencephaly and body stalk anomalies.
(2) Malformations resulting in severe dysfunction or disability, i.e., complex heart malformations and abdominal wall defects.
(3) Malformations resulting in mild dysfunction, i.e., ventricular septal defect (VSD) and polydactyly. Only cases with complete follow-up results were enrolled.
Single umbilical artery, aberrant right subclavicular artery, and persistent left superior vena cava were considered anatomical variants; the absence of nasal bone, tricuspid regurgitation, and choroid cyst were considered ultrasound markers, none of which were included in the structural malformations of this study. Complications specific to twins, such as twin-to-twin transfusion syndrome (TTTS) and twin reversed arterial perfusion syndrome (TRAPs), were not included in the category of structural malformations.
The data was analyzed by SPSS 21.0 (IBM Corp., Chicago, IL, USA). The two-tailed Fisher’s exact test was used to compare the prevalence of structural malformations between fetuses with increased NT (above the 95th percentile) and those with normal NT.
Among the 7523 fetuses screened for NT (6376 singletons and 569 twins), 147 were lost to follow-up (133 singletons and 7 twins), with the results of 7376 fetuses analyzed. One hundred nineteen cases had structural malformations, resulting in a prevalence of 1.61% (119/7376). The overall detection rate of prenatal ultrasound detecting fetal structural malformations was 79.8% (95/119). Notably, 24 fetuses with structural malformations were only identified through routine neonatal examination after being missed during prenatal ultrasound screening. Among the 119 cases, cardiac abnormalities were the most common (35/119, 29.4%), followed by genitourinary system abnormalities (14/119, 11.76%) and skeletal system abnormalities (13/119, 10.92%). Fig. 1 illustrates the timing of the abnormalities detected during screening.
 Fig. 1.
Fig. 1.Flowchart of the study population undergoing routine prenatal screening at our center.
In this study, structural malformations were first detected in 53.8% (64/119), 24.4% (29/119), and 1.68% (2/119) of fetuses during the first, second, and third trimesters, respectively. Four cases of suspected VSD in early pregnancy were later found to be normal. FTS showed a sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of 54.2%, 99.9%, 94.1%, and 99.3%, respectively (Table 1).
| Structural abnormalities (+) | Structural abnormalities (–) | Total | |
| FTS (+) | 64 | 4 | 68 |
| FTS (–) | 55 | 7253 | 7308 |
| Total | 119 | 7258 | 7376 |
Sensitivity: 0.941 (95% CI: 0.884–0.999); specificity: 0.992 (95% CI: 0.991–0.995);
PPV: 0.542 (95% CI: 0.451–0.634); NPV: 0.999 (95% CI: 0.999–1.000); odd ratio: 2149.3
(95% CI: 755.89–6111.5); relative risk: 127.37 (95% CI: 67.01–167.24);
FTS, first-trimester ultrasound screening; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value.
Furthermore, FTS detected 57.1% (20/35) of all congenital cardiac malformations, including 100% detection rates for tetralogy of Fallot (TOF) (3/3) right aortic arch (RAA) (1/1) (Fig. 2), hypoplastic left heart syndrome (HLHS) (1/1) (Fig. 3), atrioventricular septal defect (AVSD) (3/3) (Fig. 4), and heterotaxy syndrome (1/1).
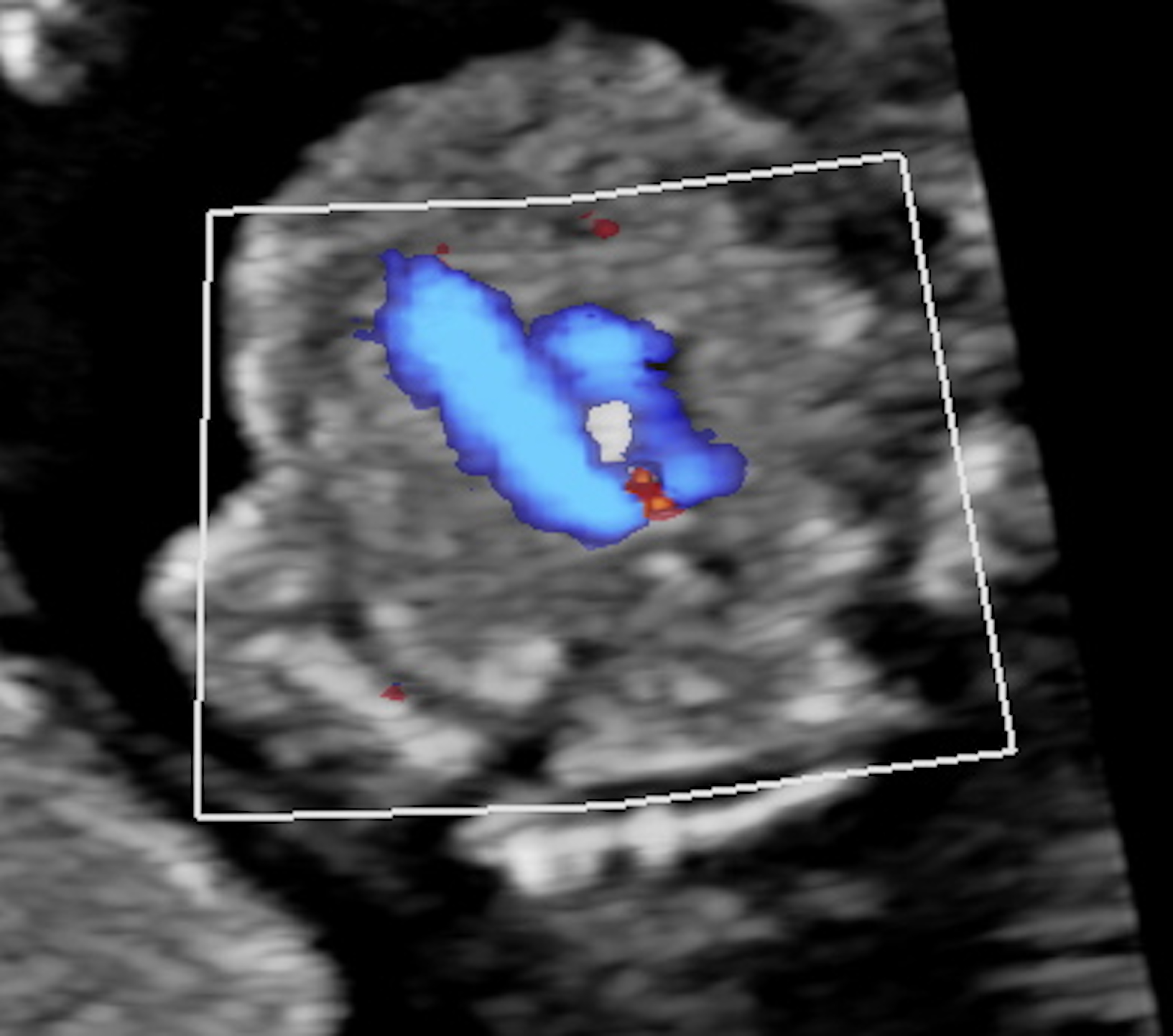 Fig. 2.
Fig. 2.RAA in a 12w6d fetus. The color doppler showing the typical “U” shape connection of the aortic transverse arch and the ductus arteriosus on the 3VT view. RAA, right aortic arch; 3VT, three-vessel-tracheal.
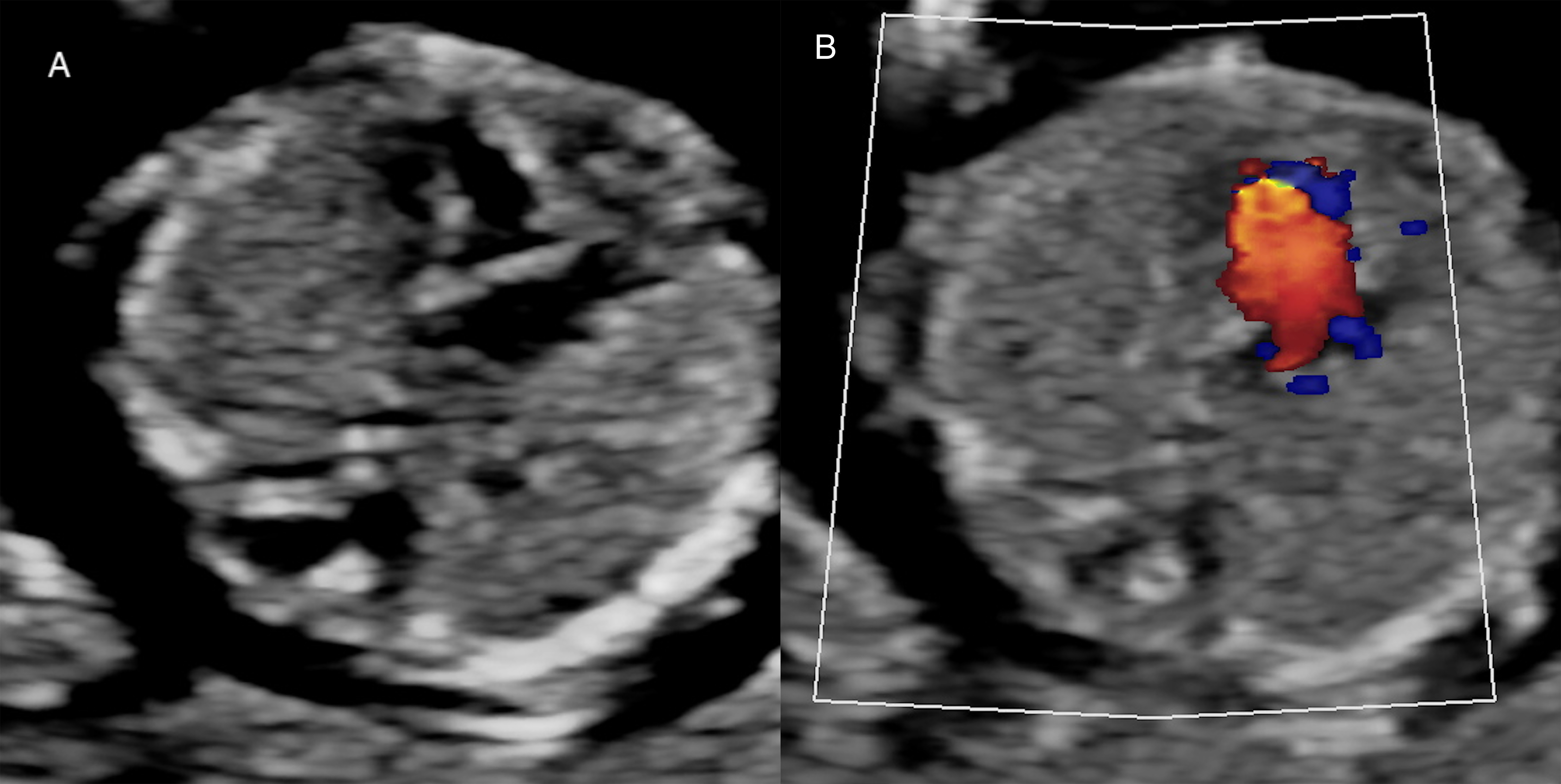 Fig. 3.
Fig. 3.HLHS in a 13w1d fetus. (A) The 4-chamber view showing the significantly reduced left atrium and left ventricle. (B) Color Doppler showing only blood flow from the right atrium to right ventricle on the 4-chamber view. HLHS, hypoplastic left heart syndrome.
 Fig. 4.
Fig. 4.AVSD in a 12w5d fetus. (A) The 4-chamber view showing the disappearance of the heart cross. (B) Color Doppler sonogram showing a single blood flow on the 4-chamber view. AVSD, atrioventricular septal defect.
For central nervous system (CNS) malformations, FTS detected 50% (3/6) of cases, 100% for holoprosencephaly (2/2) (Fig. 5) and encephalocele (1/1) (Fig. 6).
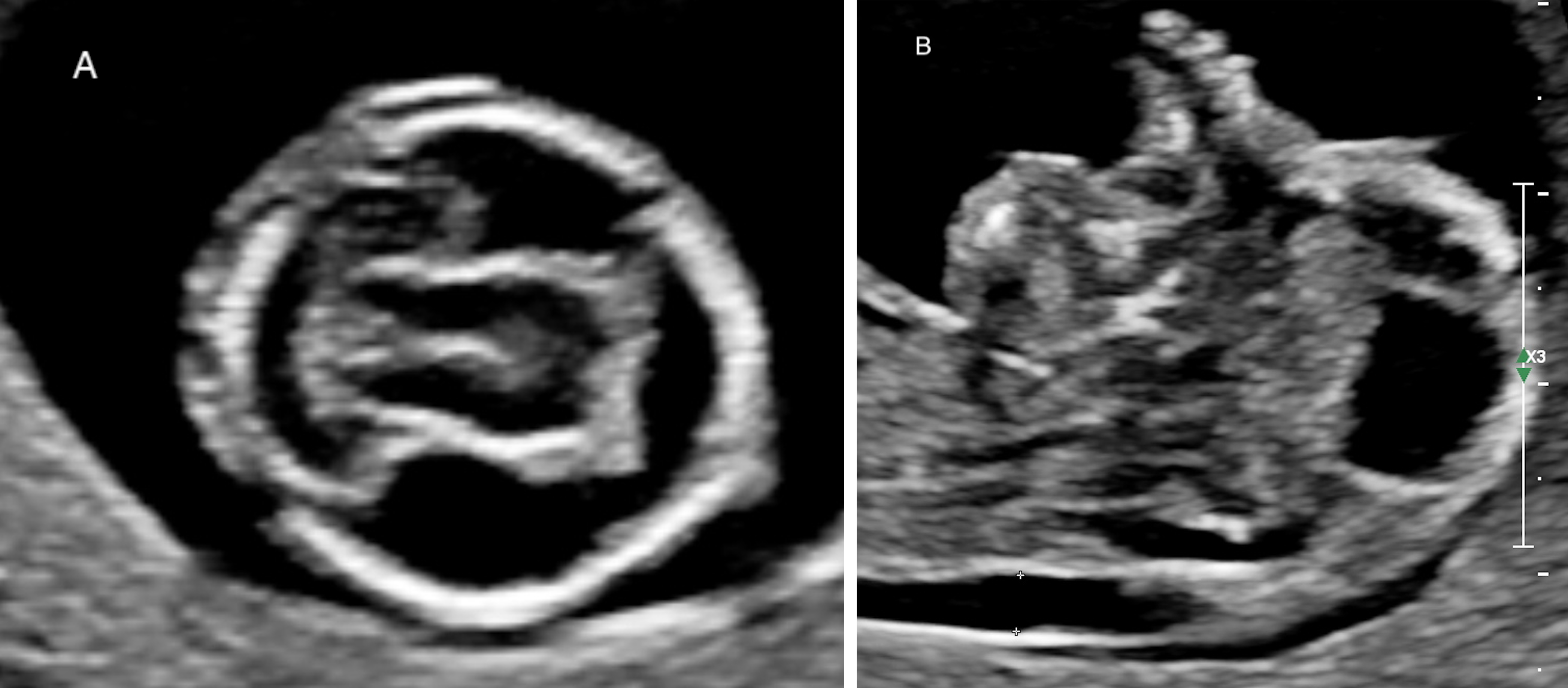 Fig. 5.
Fig. 5.Alobar holoprosencephaly in a 13w4d fetus. (A) Transverse section showing a single ventricular cavity in the fetal head. (B) Midsagittal section showing the abnormal face.
 Fig. 6.
Fig. 6.Encephalocele in a 13w3d fetus. The arrowhead showing the skull defect and bulging of cranial contents.
Similarly, FTS detected 38.5% (5/13) of all facial malformations, with 71.4% (5/7) of cleft lip and palate (CLP) cases (Fig. 7) recognized during early pregnancy.
 Fig. 7.
Fig. 7.CLP detected by RNT plane in a 12w3d fetus. (A) The coronal section showing a disruption of the maxillary. (B) The coronal section showing a disruption of the upper lip. CLP, cleft lip and palate; RNT, retronasal triangle plane.
FTS revealed thorax malformations in 33.3% (1/3) of cases, with a higher incidence of 50% (1/2) in cases of congenital diaphragmatic hernia (CDH) (Fig. 8).
 Fig. 8.
Fig. 8.CDH in a 13w2d fetus. (A) The transverse section showing the stomach in the thorax with the heart shifted to right. (B) The sagittal view showing the stomach located above the diaphragm. CDH, congenital diaphragmatic hernia.
In addition, FTS identified 83.3% (10/12) of fetuses with multisystem malformations and 66.7% (4/6) of fetal syndromes.
All these structural malformations identified by FTS were displayed in Table 2.
| Structural abnormalities | Total | Increased NT | Detection, n | Outcome, n | ||||||
| n | N (%) | T1 (DR, %) | T2 | T3 | Postnatal | TOP (%) | Live birth (%) | |||
| Central nervous system | 6 | 2 (33.3) | 3 (50.0) | 3 | 0 | 0 | 6 (100) | 0 | ||
| Always detectable | Holoprosencephaly | 2 | 0 | 2 (100) | 0 | 0 | 0 | 1 | 0 | |
| Encephalocele | 1 | 1 | 1 (100) | 0 | 0 | 0 | 1 | 0 | ||
| Never detectable | Arachnoid cyst | 1 | 1 | 0 (0) | 1 | 0 | 0 | 1 | 0 | |
| ACC | 1 | 0 | 0 (0) | 1 | 0 | 0 | 1 | 0 | ||
| Hydrocephalocele | 1 | 1 | 0 (0) | 1 | 0 | 0 | 1 | 0 | ||
| Face | 13 | 1 (7.7) | 5 (38.5) | 4 | 0 | 4 | 7 (53.8) | 6 (46.2) | ||
| Sometimes detectable | CLP | 7 | 1 | 5 (71.4) | 2 | 0 | 0 | 7 | 0 | |
| Cleft lip only | 2 | 0 | 0 (0) | 2 | 0 | 0 | 0 | 2 | ||
| Never detectable | Anomalies of ears | 4 | 0 | 0 (0) | 0 | 0 | 0 | 0 | 4 | |
| Congenital heart defects | 35 | 14 (40) | 20 (57.1) | 7 | 0 | 8 | 23 (65.7) | 12 (34.3) | ||
| Always detectable | TOF | 3 | 1 | 3 (100) | 0 | 0 | 0 | 3 | 0 | |
| RAA | 1 | 1 | 1 (100) | 0 | 0 | 0 | 1 | 0 | ||
| HLHS | 1 | 0 | 1 (100) | 0 | 0 | 0 | 1 | 0 | ||
| Heterotaxy syndrome | 1 | 0 | 1 (100) | 0 | 0 | 0 | 1 | 0 | ||
| Unknown cardiac abnormalities | 2 | 2 | 2 (100) | 0 | 0 | 0 | 2 | 0 | ||
| AVSD | 3 | 3 | 3 (100) | 0 | 0 | 0 | 3 | 0 | ||
| Sometimes detectable | Complex heart malformation | 9 | 4 | 7 (77.8) | 2 | 0 | 0 | 9 | 0 | |
| DORV | 2 | 0 | 1 (50) | 1 | 0 | 0 | 2 | 0 | ||
| VSD | 9 | 2 | 1 (11.1) | 3 | 0 | 5 | 0 | 9 | ||
| Never detectable | Cardiac tumor | 1 | 0 | 0 (0) | 1 | 0 | 0 | 1 | 0 | |
| ASD | 3 | 1 | 0 (0) | 0 | 0 | 3 | 0 | 3 | ||
| Thorax | 3 | 1 (33.3) | 1 (33.3) | 2 | 0 | 0 | 2 (66.7) | 1 (33.3) | ||
| Sometimes detectable | CDH | 2 | 1 | 1 (50) | 1 | 0 | 0 | 2 | 0 | |
| Never detectable | Congenital chylothorax | 1 | 0 | 0 (0) | 1 | 0 | 0 | 0 | 1 | |
| Gastrointestinal tract | 2 | 0 (0) | 0 (0) | 1 | 0 | 1 | 0 | 2 (100) | ||
| Never detectable | Situs inversus | 1 | 0 | 0 (0) | 1 | 0 | 0 | 0 | 1 | |
| CBA | 1 | 0 | 0 (0) | 0 | 0 | 1 | 0 | 1 | ||
| Genitourinary system | 14 | 2 (14.3) | 2 (14.3) | 8 | 1 | 3 | 7 (50) | 7 (50) | ||
| Always detectable | Megalocystis | 2 | 1 | 2 (100) | 0 | 0 | 0 | 2 | 0 | |
| Never detectable | MCDK | 5 | 0 | 0 (0) | 5 | 0 | 0 | 2 | 3 | |
| Obstructive renal dysplasia | 1 | 1 | 0 (0) | 1 | 0 | 0 | 1 | 0 | ||
| Unilateral renal agenesis | 1 | 0 | 0 (0) | 1 | 0 | 0 | 1 | 0 | ||
| Hypospadias | 1 | 0 | 0 (0) | 0 | 0 | 1 | 0 | 1 | ||
| Pelvic kidney | 1 | 0 | 0 (0) | 1 | 0 | 0 | 0 | 1 | ||
| Hydronephrosis ( |
1 | 0 | 0 (0) | 0 | 1 | 0 | 0 | 1 | ||
| Abdominal wall | 1 | 1 (100) | 1 (100) | 0 | 0 | 0 | 1 (100) | 0 | ||
| Always detectable | Omphalocele | 1 | 1 | 1 (100) | 0 | 0 | 0 | 1 | 0 | |
| Abdominal/Pelvic mass | 1 | 0 | 0 (0) | 0 | 1 | 0 | 0 | 1 (100) | ||
| Never detectable | Adrenal cyst | 1 | 0 | 0 (0) | 0 | 1 | 0 | 0 | 1 | |
| Limbs and skeleton | 13 | 5 (38.5) | 5 (38.5) | 3 | 0 | 5 | 6 (46.2) | 7 (53.8) | ||
| Always detectable | Limb reduction defects | 2 | 2 | 2 (100) | 0 | 0 | 0 | 2 | 0 | |
| Severe short limbs | 1 | 1 | 1 (100) | 0 | 0 | 0 | 1 | 0 | ||
| Hemivertebrae | 1 | 0 | 1 (100) | 0 | 0 | 0 | 1 | 0 | ||
| Sometimes detectable | Club foot | 2 | 1 | 1 (50) | 1 | 0 | 0 | 0 | 2 | |
| Never detectable | Short femur | 1 | 0 | 0 (0) | 1 | 0 | 0 | 1 | 0 | |
| Achondroplasia | 1 | 1 | 0 (0) | 1 | 0 | 0 | 1 | 0 | ||
| Polydactyly | 2 | 0 | 0 (0) | 0 | 0 | 2 | 0 | 2 | ||
| Syndactyly | 1 | 0 | 0 (0) | 0 | 0 | 1 | 0 | 1 | ||
| Anomalies of the hand and foot joints | 1 | 0 | 0 (0) | 0 | 0 | 1 | 0 | 1 | ||
| Hip dysplasia | 1 | 0 | 0 (0) | 0 | 0 | 1 | 0 | 1 | ||
| Others | 13 | 13 (100) | 13 (100) | 0 | 0 | 0 | 13 (100) | 0 | ||
| Always detectable | Hygroma, fetal hydrops | 13 | 13 | 13 (100) | 0 | 0 | 0 | 13 | 0 | |
| Multiple defects | 12 | 7 (58.3) | 10 (83.3) | 1 | 0 | 1 | 10 (83.3) | 2 (16.7) | ||
| Always detectable | Omphalocele, hemivertebrae | 1 | 0 | 1 (100) | 0 | 0 | 0 | 0 | 1 | |
| Meningoencephaloceles, situs inversus viscerum | 1 | 1 | 1 (100) | 0 | 0 | 0 | 1 | 0 | ||
| Hygroma, absence of radius, abnormal hands position, VSD | 1 | 1 | 1 (100) | 0 | 0 | 0 | 1 | 0 | ||
| Heart defects, holoprosencephaly | 1 | 1 | 1 (100) | 0 | 0 | 0 | 1 | 0 | ||
| Hygroma, segmental spinal dysplasia, short limbs | 1 | 1 | 1 (100) | 0 | 0 | 0 | 1 | 0 | ||
| Hygroma, meningoencephaloceles, caudal regression, AVSD, absence of bilateral radius | 1 | 1 | 1 (100) | 0 | 0 | 0 | 1 | 0 | ||
| Hygroma, absence of left radius | 1 | 1 | 1 (100) | 0 | 0 | 0 | 1 | 0 | ||
| Meningoencephaloceles, facial abnormalities | 1 | 0 | 1 (100) | 0 | 0 | 0 | 1 | 0 | ||
| Meningoencephaloceles, CHD, abnormal ductus venosus, absence of right radius, abnormal hands position, intraabdominal calcification | 1 | 0 | 1 (100) | 0 | 0 | 0 | 1 | 0 | ||
| Left lung hypoplasia, VSD | 1 | 0 | 1 (100) | 0 | 0 | 0 | 1 | 0 | ||
| Never detectable | COA, pelvic kidney | 1 | 0 | 0 (0) | 0 | 0 | 1 | 0 | 1 | |
| Hydrocephalus, bilateral MCDK | 1 | 0 | 0 (0) | 1 | 0 | 0 | 1 | 0 | ||
| Syndrome | 6 | 2 (33.3) | 4 (66.7) | 0 | 0 | 2 | 4 (66.7) | 2 (33.3) | ||
| Always detectable | Body stalk anomaly | 1 | 1 | 1 (100) | 0 | 0 | 0 | 1 | 0 | |
| Caudal regression syndrome | 1 | 1 | 1 (100) | 0 | 0 | 0 | 1 | 0 | ||
| Larsen syndrome | 1 | 1 | 1 (100) | 0 | 0 | 0 | 1 | 0 | ||
| Sometimes detectable | Pentalogy of Cantrell | 2 | 0 | 1 (50) | 0 | 0 | 1 | 1 | 1 | |
| Never detectable | Pierre Robin syndrome | 1 | 0 | 0 (0) | 0 | 0 | 1 | 0 | 1 | |
| Total | 119 | 48 (40.3) | 64 (53.8) | 29 (24.3) | 2 (1.7) | 24 (20.2) | 79 (66.4) | 40 (33.6) | ||
NT, Nuchal translucency; DR, Detection rate; TOP, Termination of pregnancy; VSD, Ventricular septal defects; TOF, Tetralogy of Fallot’s disease; RAA, Right aortic arch; HLHS, Hypoplastic left heart syndrome; AVSD, Atrioventricular septal defect; CLP, Cleft lip and palate; CDH, Congenital diaphragmatic hernia; DORV, Double-outlet right ventricle; ACC, Agenesis of corpus callosum; ASD, Atrial septal defects; CBA, Congenital biliary atresia; MCDK, Multicystic dysplastic kidney; CHD, Congenital heart disease; COA, Coarctation of the aorta.
Forty-eight (48/452, 10.6%) fetuses with thickened NT (above the 95th percentile) demonstrated structural malformations. The prevalence of structural abnormalities was significantly higher in fetuses with increased NT (48/452, 10.6%) than in those with normal NT (71/6924, 1.0%) (p = 0.000) (Table 3).
| Structural abnormalities (+) | Structural abnormalities (–) | Total | |
| Increased NT (+) | 48 | 404 | 452 |
| Increased NT (–) | 71 | 6853 | 6924 |
| Total | 119 | 7257 | 7376 |
NT, nuchal translucency.
In recent years, various studies have reported detection rates of fetal structural malformations in early pregnancy, with an average detection rate of approximately 50% [8, 9]. The rates vary depending on the studied populations, the criteria for inclusion of malformations, and the existence of detailed standard protocols. Yimei Liao et al. [10] reported a more recent detection rate of 43.1% in the first trimester scan. Our study, which included a non-selective population and applied an established standard protocol, detected structural malformations in early pregnancy at a rate of 53.8% (64/119), regardless of chromosomal aberrations.
The performance of FTS varied in different studies. In Fernando Felix Dulgheroff et al. [11] study, the sensitivity, specificity, PPV, and NPV of first-trimester ultrasound were reported as 14.06%, 98.65%, 39.13%, and 94.90%, respectively. The corresponding values in our study were as follows: 54.2%, 99.9%, 94.1%, and 99.3%, respectively. The FTS utilizing a standardized scanning protocol in our study had significantly higher sensitivity and PPV, suggesting a better performance in detecting malformations.
In the study by Syngelaki et al. [12], acrania, ectopia cordis, alobar holoprosencephaly, exomphalos, gastroschisis, tricuspid or pulmonary atresia, pentalogy of Cantrell, and body-stalk anomalies were all detected in the first trimester, indicating the high sensitivity of early screening. Ana Maria Vayna et al. [13] identified all cases of AVSD, right atrial isomerism, double-outlet right ventricle (DORV), megacystis, persistent cloaca, and aplasia/hypoplasia of radius/ulna. Similarly, our study detected all alobar holoprosencephaly, encephalocele, exomphalos, hygroma, TOF, RAA, HLHS, AVSD, heterotaxy syndrome, megalocystis cases, limb reduction defects, severe short limb, and hemivertebrae cases. Due to a previous ultrasound examination prior to the first trimester NT screening in our center, no acrania cases were detected. The detection rate of the pentalogy of Cantrell and DORV was 50% (1/2) in our study, further highlighting the complexity of these conditions. One case of DORV was not proven until the mid-trimester due to poor image quality. Our study demonstrated a remarkable 100% detection rate for syndromes such as caudal regression syndrome and Larsen syndrome.
Furthermore, our investigation also revealed a high detection rate of
Our study found that certain abnormalities, including agenesis of the corpus callosum (ACC), arachnoid cyst, hydrocephalus, cardiac tumor, atrial septal defects (ASD), congenital chylothorax, congenital biliary atresia (CBA), as well as genitourinary system abnormalities such as multicystic dysplastic kidney (MCDK) and unilateral renal agenesis, were not detectable in the first trimester. This may be due to their late onset and subtle manifestation during this period. However, our retrospective analysis revealed that 1 case of situs inversus, initially missed by FTS, could have been detected.
Although deformities relating to the corpus callosum and the posterior fossa were conventionally considered undetectable, several indirect signs have been reported to predict ACC [21, 22, 23, 24] and posterior fossa malformations [22, 23, 24]. Also, for early detection of ventriculomegaly, various indicators have been extensively explored [25, 26]. Through our study, we uncovered that the fetal kidneys are more discernible on the coronal plane than on the transverse plane, especially with the augmentation of ultrasound instrument gain. Nevertheless, the identification of MCDK in the first trimester remains unattainable, as corroborated by recent studies [10, 12]. Assessing the urinary tract poses a challenge, as signs indicative of urinary tract deformities are not discernible in early pregnancy. As our study has shown, the presence of hydronephrosis and obstructive renal dysplasia suggests a gradual progression during fetal development.
Twenty-four cases with missed prenatal diagnosis exhibited deformities across various systems, including ear abnormalities, VSD and ASD, hypospadias and ambiguous genitalia, polydactyly, syndactyly, abnormalities of hand and foot joints, and hip dysplasia in the limbs and skeleton system. Additionally, 1 case exhibited multiple defects, namely coarctation of the aorta and pelvic kidney, while another case was diagnosed with Pierre Robin syndrome (PRS). Fortunately, none of these deformities proved fatal, and the majority can be surgically treated barring PRS. Our examination of the initial images in the PRS case revealed an overlooked sign, that being a small mandible.
Four cases of VSD diagnosed by FTS were later confirmed as being normal. Two explanations were proposed: either the VSD was present in the first trimester but healed spontaneously or the VSD was misdiagnosed altogether. Considering the small size of the heart and the limitations of two-dimensional and color imaging, diagnosis of VSD in early pregnancy requires careful consideration.
Increased NT thickness, equal to or above the 95th percentile, has been associated with high risk of aneuploidies [27, 28, 29], often accompanied by structural abnormalities. In chromosomally normal fetuses, thickened NT can signal potential structural defects in the cardiovascular, gastrointestinal, or musculoskeletal systems, demanding a thorough anatomical assessment [30, 31]. Our analysis of 452 fetuses with thickened NT revealed structural malformations in 10.6% (48/452) of cases, significantly higher compared to fetuses with normal NT (71/6924, 1.0%, p = 0.000). Congenital heart defects (CHD) had the highest incidence of NT thickening (14/35, 40%), followed by limb and skeletal defects (5/13, 38.5%), CNS defects (2/6, 33.3%), and fetal syndromes (2/6, 33.3%). With a higher incidence of 58.5% (7/12), multiple defects indicate a potential necessity for further screening for structural malformations. Therefore, an increased NT measurement could be a valuable indicator for directing such screening efforts.
Several limitations of this study should be highlighted. Primarily, due to its retrospective nature and moderate sample size, the findings may not be fully representative. Additionally, the use of solely transabdominal ultrasound examination may have resulted in a lower detection rate compared to using a combination of transvaginal and transabdominal ultrasound. Moreover, a significant number of pregnant patients with fetal malformations underwent fetal genetic testing and exploration of the relationship between malformations and genetic results shall be pursued in subsequent studies.
Apart from the NT measurements, our study found that standardized FTS can identify over 50% of the structural anomalies present. Furthermore, the use of a standardized screening protocol enhances the detection of abnormalities that may not be readily discernable during the early stages of pregnancy, particularly in the identification of complicated cardiac malformations, CLP, and severe deformities. The elevated NT levels during the early stages of pregnancy serve as an indication for more detailed structural screening. In addition, the use of standardized FTS provides earlier opportunities for genetic testing, specialist imaging, prognostic information, and management discussions. Nonetheless, screening during the second and third trimesters remains essential, as some malformations may have a delayed onset and certain organs develop later in pregnancy.
FTS, First-trimester ultrasound screening; NT, Nuchal translucency; VSD, Ventricular septal defect; PPV, Positive predictive value; NPV, Negative predictive value; IQR, Interquartile range; CRL, Crown-rump length; FMF, Fetal Medicine Foundation; DV, Ductus venosus; ISUOG, International Society of Ultrasound in Obstetrics and Gynecology; LVOT, Left ventricular outflow tract; 3VT, three-vessel-tracheal; TOF, Tetralogy of Fallot’s disease; RAA, Right aortic arch; HLHS, Hypoplastic left heart syndrome; AVSD, Atrioventricular septal defect; CNS, Central nervous system; CLP, Cleft lip and palate; CDH, Congenital diaphragmatic hernia; DORV, Double-outlet right ventricle; RNT, Retronasal triangle plane; MG, Maxillary gap; SMA, Superior mesenteric artery; ACC, Agenesis of corpus callosum; ASD, Atrial septal defects; CBA, Congenital biliary atresia; MCDK, Multicystic dysplastic kidney; PRS, Pierre Robin syndrome.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
LS drafted the whole manuscript. XD and LY designed the research study and revised the manuscript. LS, CJ, QP, JZ, ZY and CL performed the data collection. JS and LS completed the statistical analysis of the data. All authors contributed to editorial changes in the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
This study was approved by the Ethics Committee of Suzhou Municipal Hospital (K2016038). Informed consent was obtained from all individual participants included in the study.
The authors thank all the doctors, nurses in the Center for Medical Ultrasound, the affiliated Suzhou Hospital of Nanjing Medical University, Suzhou Municipal Hospital for their help in collecting data. Special thanks to women who participated in this study for partnering with us in providing the data used for this study.
This study was sponsored by Chinese Multi Centered Clinical Trial (ChiCTR-SOC-17010976), Suzhou Gusu Health Talents Program (GSWS2019006, GSWS2020055), Jiangsu Provincial Maternal and Child Health Scientific Project (F202044), Suzhou “Rejuvenating Health through Science and Education” Youth Science Project (KJXW2021032) and Scientific Program from Gusu School, Nanjing Medical University (GSKY20210232).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
