- Academic Editor
Background: Uterine compression methods reduce the amount of postpartum bleeding. In our study, we investigated the effect of fundal pressure, which will be created by a sandbag placed on the abdomen, on reducing post-cesarean bleeding. Methods: A total of 482 patients who delivered by cesarean section (CS) in the Obstetrics Clinic of Fırat University Faculty of Medicine between January 2021 and December 2021 were included in this prospective, randomized, single-center study. There were two groups: control group (n = 246), weighted group (n = 236). A sandbag weighing approximately 3 kg was used as a fundal compression tool. Hemoglobin (Hb) and hematocrit (Hct) concentrations and amount of vaginal bleeding were determined preoperatively and at the postoperative 8th and 24th hours. In addition, the time of milk coming from the breast and visual analogue scale (VAS) were evaluated. Results: The postoperative Hb value at 24 hours was significantly lower in weighted group compared to control group. The estimated amount of postoperative bleeding (based on the number of pads) was higher in weighted group compared to control group. The time to onset of milk production from the breast at the postoperative 8th hour was significantly longer in weighted group compared to control group. Postoperative VAS scores at 24 hours were significantly higher in weighted group compared to control group. Conclusions: Applying fundal pressure by using a sandbag from the abdominal route seems ineffective in reducing the amount of bleeding after CS. It may even increase the amount of bleeding. Clinical Trial Registration: The study was registered at https://clinicaltrials.gov/, registration number: NCT06005831.
A little more than a century ago, cesarean section (CS), avoided due to the alarming mortality rate, is now the mode of delivery for one in three women in the United States [1]. This rate rises to four out of five women in some places in the world [2]. According to 2017 data, CS rates in Turkey were reported as 51.2%. CS rates are higher for all Robson groups than for the World Health Organization (WHO) Multi-country Survey Reference (MCS) population [3]. Even under optimal conditions, women who have a CS lose more blood than those who have a vaginal delivery (VD) and are at higher risk for postpartum hemorrhage (PPH) [4, 5]. PPH is an important cause of maternal morbidity, including prolonged hospital stay, blood transfusion, and hysterectomy, and is the leading cause of maternal death worldwide [6, 7, 8].
Cesarean section is an accepted risk factor for PPH [9]. Traditionally, PPH has been defined as blood loss exceeding 500 mL after vaginal delivery or 1000 mL after CS. The American Society of Obstetrics and Gynecology recommended the use of different criteria in the diagnosis of PPH [10]. These include a hematocrit decrease of more than 10%, the need for blood transfusion, and hemodynamic instability [11, 12]. Uterine atony is responsible for 50–80% of all PPH cases [8, 13].
The third stage of labor, which begins with the birth of the fetus, is an important time period to prevent postpartum hemorrhages. The first step in the management of postpartum bleeding is the use of uterine uterotonics such as oxytocin, ergot derivatives and misoprostol, and bimanual compression of the uterus [14].
In our study, we investigated the effect of fundal pressure created by the sandbag placed on the abdomen as a way of applying compression to the uterus on reducing post-cesarean bleeding.
A total of 482 patients who gave birth by CS in the Obstetrics Clinic of Fırat University Faculty of Medicine between 1 January 2021 and 31 December 2021 were included in the study. Approval was obtained from the Ethics Committee of Fırat University for this prospective randomized single-center study (Ethics committee date = 15.10.2020; decision no = 2020/14-02). The 482 patients who had CS were divided into two groups, control group (n = 246), weighted group (n = 236) was randomized. Each patient was followed up in our obstetrics clinic until discharge from the hospital after surgery and the data were analyzed. The same techniques were used in all surgical procedures in order not to create differences in terms of surgical techniques and operation times that may affect intraoperative bleeding. However, to minimize inter-operator variations, surgical procedures were performed by operators with sufficient surgical experience.
The standard sandbag used in our clinic was 20
 Fig. 1.
Fig. 1.The standard sandbag used in our clinic. 20
It is a prospective, randomized, controlled single-center study.
All cases with CS who had a live pregnancy after 24 weeks of gestation were included in the study.
Patients who did not give consent, those with placental invasion anomaly, diagnosed with preeclampsia, placenta previa cases, hypertensive pregnant women receiving antihypertensive therapy, diabetic pregnant women, multiple pregnancies, major hepatic, cardiac, renal, respiratory disorders and deep vein thrombosis during pregnancy, and those receiving anticoagulant therapy were excluded from the study. In addition, patients who developed atony and uterine rupture in the operating room, suspected placental invasion, uterine balloon tamponade, arterial embolization, uterine and hypogastric artery ligation, and uterine compression sutures were also excluded from the study. In addition, cases whose intraoperative blood loss was estimated to be over 1000 mL during CS and who received intraoperative blood transfusion were excluded from the study in order not to cause errors in postoperative hemoglobin results.
For randomization, patients were fully informed about the study while preparing for CS. Sandbags were placed in all cases included in the study after meeting the study criteria for between 1 January 2021 and 30 June 2021. Sandbags were not placed in the cases who met the study criteria and were included in the study between 1 July 2021 and 31 December 2021.
Qualified participants who gave informed consent to participate in the study were randomly assigned to groups. Visual analog scale (VAS) was used for postoperative pain scoring at the 8th and 24th postoperative hours. The patient included in the study was randomized into control group or weighted group by another assistant doctor who did not participate in the CS and did not know about the patient.
All cases were given 10 IU of oxytocin (Synpitan® forte ampule 5 IU,
Deva, Istanbul, Turkey) intravenous (IV) during CS, after
prophylactic antibiotic therapy and placenta separation. After CS,
40 IU of oxytocin was administered intravenously in 500 mL
saline and at an infusion rate of 125 mL/hour. At the same time,
0.2–0.4 µg methylergonovine maleate (Methergine®
ampule 0.2 µmg, Sandoz, Istanbul, Turkey) was given
intravenously/intramuscularly in cases without hypertension. All patients were
treated according to the postoperative protocol for CS performed in our
obstetrics clinic. At the 8th and 24th hours postoperatively, the researchers
determined the patient’s hemoglobin (Hb) and hematocrit (Hct) concentrations and
the amount of vaginal bleeding. In addition, the time of milk coming from the
breast and VAS were performed. Patients with a postoperative Hb value of
Postoperative blood loss was determined by monitoring the pad and measuring the
hemoglobin values at the 8th [16] and 24th hours in all cases. Standard pads
measuring 10
Statistical analysis of the data was performed using SPSS 21.0 (IBM Corporation,
Armonk, NY, USA) package program. Numerical data were expressed as mean
There was no significant difference between the groups in terms of age, parity CS number and gestational week. Preoperative Hb and Hct values were similar in both groups. Postoperative Hb and Hct values were significantly lower in weighted group compared to control group. The estimated amount of postoperative bleeding (based on the number of pads) were higher in weighted group compared to control group. A total of 8 units of blood was given to 4 patients in control group and 12 units of blood was given to 5 patients in weighted group. There was no significant difference in the amount of transfusion between the two groups. The time to onset of milk production from the breast at the postoperative 8th hour was significantly longer in eighted group compared to control group (Table 1).
| Parameters | Control group | Weighted group | p value |
| (n = 246) | (n = 236) | ||
| Age (years) | 29.61 |
29.42 |
0.643 |
| Number of cesarean sections | 1.18 |
1.28 |
0.273 |
| Gestational week | 36.33 |
36.42 |
0.788 |
| Gravida | 3.42 |
3.12 |
0.171 |
| Parity | 1.82 |
1.65 |
0.313 |
| Preoperative Hb value (gr/dL) | 12.25 |
12.30 |
0.092 |
| Postoperative Hb value (gr/dL) | 12.02 |
10.95 |
0.0113* |
| Preoperative Hct value (%) | 36.87 |
36.81 |
0.8428 |
| Postoperative Hct value (%) | 34.77 |
33.12 |
|
| Postoperative amount of bleeding (mL) | 114.28 |
124.36 |
0.040* |
| Blood transfusion rate n (%) | 4 (1.6) | 5 (2.1) | 0.474 |
| Start time of lactation (hour) | 3.47 |
4.35 |
0.041* |
*, = Compared with control group. Amount of blood transfusion and time of milk
arrival (values are given as mean
While there was no significant difference between the groups when the postoperative VAS was compared at the 8th hour, it was significantly higher in weighted group compared to the control group at the 24th hour (Table 2).
| Visual analogue scale (VAS) scores | Control group | Weighted group | p value |
| 8th hour VAS score | 6.07 |
6.26 |
|
| 24th hour VAS score | 4.34 |
4.86 |
*, = Compared with control group. Values are given as mean
A magnetic resonance imaging (MRI) image of the uterus was given in two cases as an example of the groups that did not apply sandbags (Fig. 2) and that did (Fig. 3).
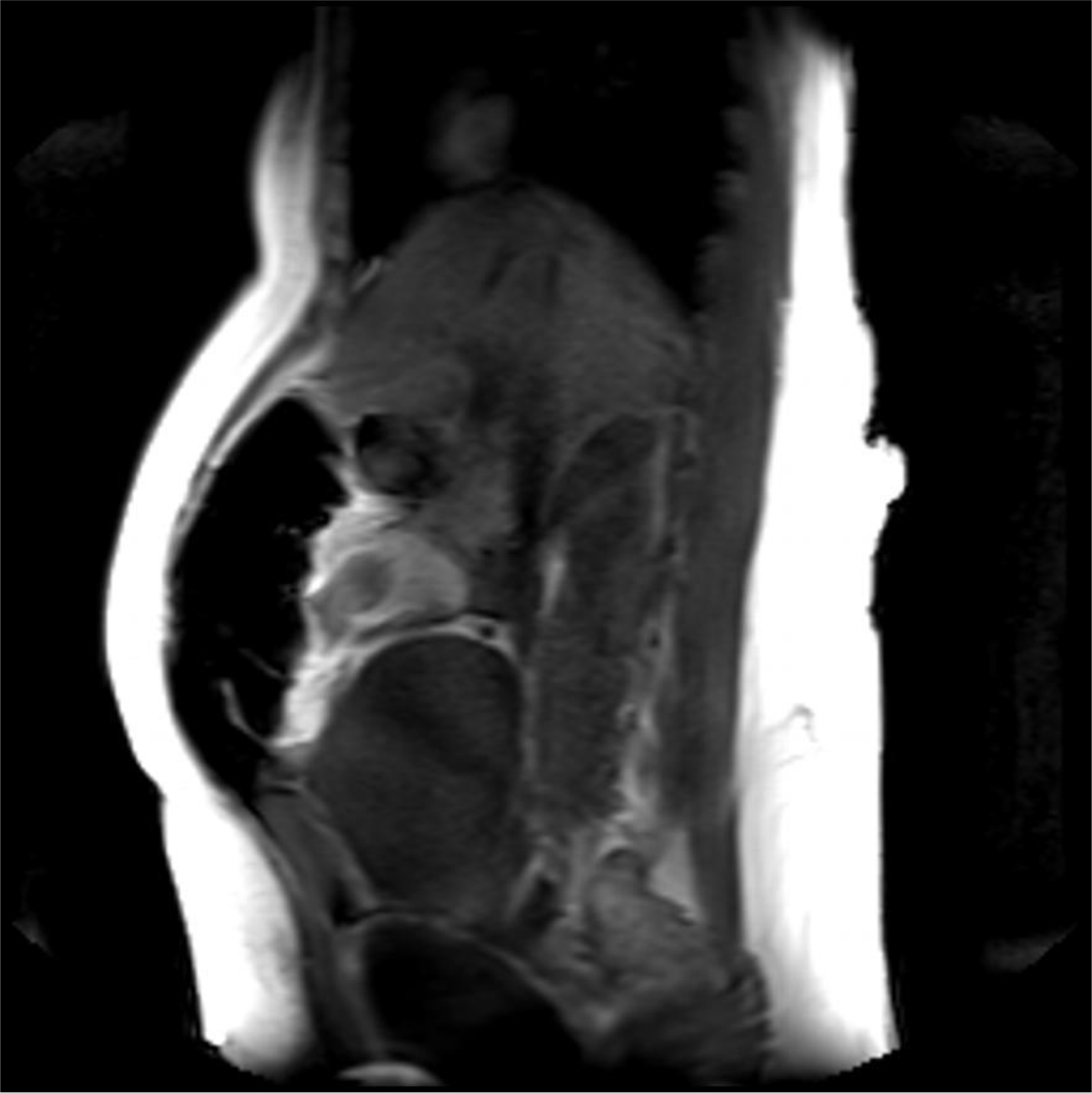 Fig. 2.
Fig. 2.Postoperative 4th hour magnetic resonance imaging (MRI) image of a patient who did not apply sandbag. It shows the uterus contracting and the anteflexio position of the uterus.
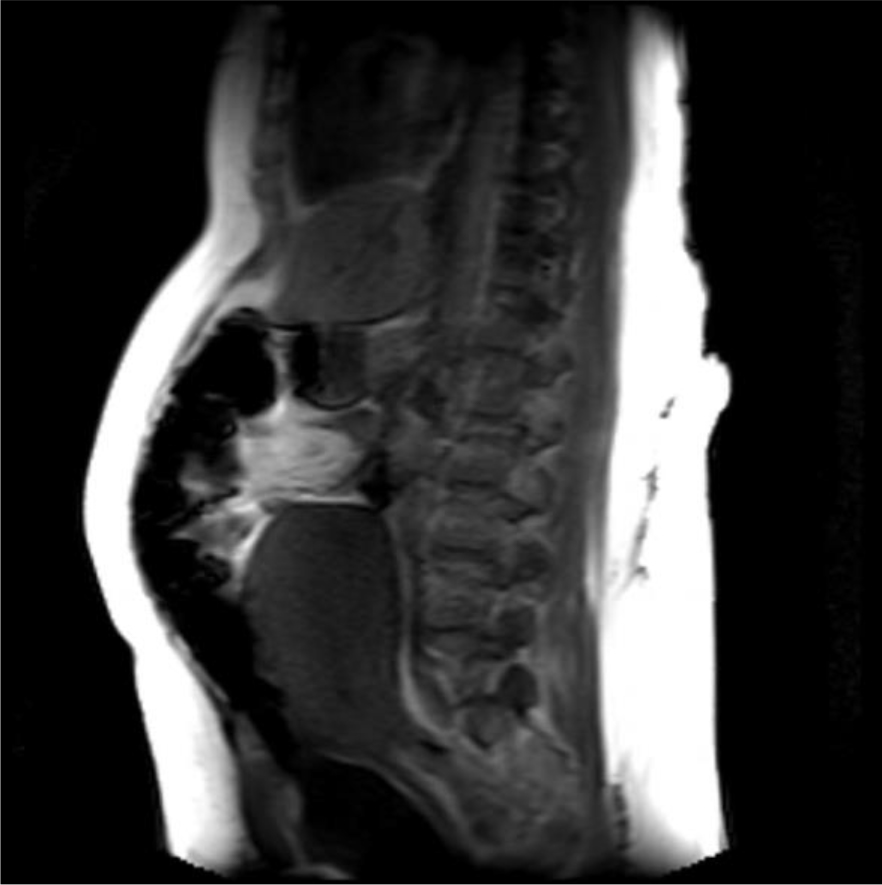 Fig. 3.
Fig. 3.Postoperative 4th hour MRI image of a case with sandbag application. It is observed that the anteflexio position of the uterus is impaired.
As a result of our study, we have seen that fundal weight application after cesarean section has no effect on reducing postpartum hemorrhage, but it causes an increase in the amount of bleeding statistically, although not clinically. We could not find any study related to the method we applied in our search from PubMed.
The physiology of the cessation of postpartum hemorrhage mainly depends on the mechanical events under the influence of hormones and causing the uterus to contract violently. When the myometrium and decidua contract after birth, they are arranged to provide bleeding control [20, 21, 22]. After the separation of the placenta, the blood vessels feeding the placental bed are compressed by the contracted myometrium, and the local and systemic hemostatic factors provide support with the coagulation cascade, and the bleeding is controlled. Dysfunction of these mechanisms or the occurrence of trauma causes postpartum hemorrhage [23, 24]. The American College of Obstetricians and Gynecologist defines early (primary) postpartum hemorrhage as the signs and symptoms of hypovolemia, including a total blood loss of 1000 mL or more within 24 hours after birth, or intrapartum loss with accompanying blood loss [25].
In late pregnancy, the enlarged uterus in the supine position puts pressure on the inferior vena cava and abdominal aorta. As a result of this compression, uterine blood flow is reduced. In the supine position, this compression is intensified and can result in a woman’s blood flow being reduced by up to 85% through the inferior vena cava and up to 30% through the aorta [26, 27, 28]. Based on this theory, we hypothesized that fundal pressure, which we apply abdominally after CS, mimics the uterus of a term pregnant and has a high probability of compressing the inferior vena cava or aorta, similar to the aortocaval compression in the supine position [29]. We predicted that this pressure may also contribute to reducing the amount of bleeding after CS. Our determination of the amount of weight as 3 kg is due to the fact that it is close to the weight of a full-term baby.
In order to control uterine bleeding, bimanual uterine compression includes massaging the posterior uterus with one hand on the abdomen and massaging the anterior uterus through the vaginal route with the other hand with a fist [30]. We also massaged the uterus during CS to strengthen the contractions in all of our cases where uterine contractions were weak during CS. In patients who developed atony despite uterine massage during or at the end of the operation, atony treatment was performed in accordance with the algorithm, and some cases underwent hysterectomy. Therefore, the patients who did not develop atony in the operating room after the end of the operation, after the skin was closed, and who were taken to the bed in the obstetrics clinic in a stable manner were included in the study.
Quantitative measurement of postpartum hemorrhage begins soon after the fetus is
born and requires measuring blood loss with a calibrated drape or by weighing
blood-soaked cloths, pads, and clots [31]. In our study, we also monitored
bleeding with standard pads of 10
Factors such as the patient’s body mass index (BMI) and the patient’s individual tolerance for blood loss may influence the risk of postpartum hemorrhage. Therefore, if postpartum hemorrhage is defined only in terms of blood loss, it can be misleading. Because, even with a blood loss of more than 1000 mL due to the increased blood volume during pregnancy, the signs and symptoms of shock may not be detected in the pregnant woman. Even in these cases, tachycardia is often the only finding. This situation obscures the extent of bleeding, causing errors and difficulties in the clinical evaluation of obstetric hemorrhage [32]. However, the inability to perform laboratory tests and blood gas analyzes immediately is another reason for the delay in the diagnosis of postpartum hemorrhage. A multidisciplinary approach is required for the diagnosis of postpartum hemorrhage. Physical condition and vital signs, clinical symptoms, speed and amound of bleeding should also be considered [33].
One of the biggest problems is how to accurately measure the amount of blood lost. The limited data available on this subject is that there is no gold standard method [34]. We calculated the amount of bleeding after CS by comparing the pad follow-up and postoperative and preoperative hemoglobin and hematocrit values.
In our study, as a second theory, we thought that by applying external pressure to the uterus, bleeding after CS could be reduced. Namely, uterine massage [35] aiming to provide contraction of the uterus with fundus pressure, uterine balloon tamponade methods aiming to stop bleeding by applying internal pressure to the uterus [36, 37], uterine compression sutures that are reported to provide hemostasis with 90% success by shrinking the uterine cavity as in bimanual uterine compression (B-Lynch, Hayman, Pereira, Square suture, U sutures, Matsubara, etc.) [38]. Some of the above-mentioned methods are effective in reducing postpartum uterine bleeding by applying pressure to the uterus from the inside, some from the outside and shrinking the uterine cavity. Based on this pressure effect, we investigated its effectiveness as a method for reducing the amount of bleeding after CS by applying abdominal fundal pressure to the uterus in the first 6 hours postpartum. As a result of our study, the amount of bleeding was statistically significantly higher than in our control group. However, when we evaluated it clinically, there was no difference in terms of anemia. In our opinion, the sandbag left on the uterine fundus disrupted the anteflexion position of the uterus and brought it to the extension position. We obtained information about the position of the uterus by magnetic resonance imaging (MRI) in some of our patients who had and did not apply sandbags. As a result of MRI, we observed that the uterus lengthened towards the umbilicus and the anteflexion position was impaired, as if the uterus was in atony in the weight-applied group. According to the results of our study, sandbag application does not seem to reduce bleeding after CS. However, sandbag application may be beneficial in preventing complications such as incision bleeding, hematomas or rectus hematoma formation due to the local pressure effect on the skin and subcutaneous tissues. However, in our study cutaneous, subcutaneous or rectus hematoma was not observed in any of our patients in either group we could not comment on this issue.
In cases where we did not apply weight, we experienced that the uterus contracted like a fist. Our MRI findings showed that weight application could not have a positive effect on uterine contractions contrary to our previous thought. This position disorder and continuous pressure effect in the uterus disrupted the synchronization of uterine contractions and was not effective in reducing bleeding. Because if we think like the B-Lynch effect, while the anteflexion position of the uterus reduces bleeding, we have disrupted this position with a sandbag. Disruption of the postpartum position of the uterus may have caused dysfunction in the contraction of the spiral arteries by the myometrium by disrupting the contraction pattern of the myometrium, or it may have created a trauma effect [23, 24]. In addition, the sandbag may have caused myometrial muscle fatigue by encouraging continuous contraction of the myometrium due to uterine pressure. Zhao et al. [39] used a multifunctional airbag abdominal compression belt in the second and third stages of labor in a study. As a result of the study, the internal pressure of the belt was closely monitored and appropriately adjusted to the patient. It has been reported that the multifunctional airbag abdominal compression belt can accelerate the second and third stages of labor, prevent postpartum hemorrhage and promote natural birth. The sandbag we used in our study can look like to the multifunctional airbag abdominal pressure belt. Because the purpose of both is to apply pressure to the uterine fundus. However, the multifunctional airbag abdominal compression belt has been used to facilitate childbirth. Pressure adjustment control has also been made in this belt. Pressure setting control can be very important during labor. Because there is a fetus that may be at risk. This pressure setting can also be lowered if the fetus is in distress during delivery. However, in our sandbag application, there is no controlled pressure setting, it presses the fundus as much as the weight of the sandbag. However, since we apply the sandbag after birth, we will not have a problem such as fetal distress. As a result, it is similar to our study that the described belt application has been shown to reduce the amount of postpartum hemorrhage as well as facilitating childbirth. However, we showed that sandbag application did not reduce the amount of postpartum bleeding. The reason for this difference may be due to the fact that the belt is more applicable and pressure-adjusted and was applied during delivery. In addition, sandbag application can be considered as a primitive method. However, our sandbag application can apply uterine pressure from the abdomen in non-invasive ways and can give an idea about the construction of pressure-adjusted compressor devices.
One of the limitations of our study is that the pregnant women included in the study were between 24 weeks and 41 weeks, not grouping separately according to gestational weeks, and the inclusion of pregnant women with primary and previous CS. Another limitation is that we do not differentiate between spinal and general anesthesia in both groups. Our third limitation, it should be taken into account that the bleeding monitoring with the pad can be interpreted differently between individuals and its reliability in estimating blood loss is not very good. In this context, in order to increase our reliability in predicting blood loss, we tried to determine the amount of bleeding according to pre- and postoperative hemoglobin values, as well as hemorrhage follow-up with the pad.
The strength of our study is that contrary to expectations, we showed that the application of fundal compression did not reduce the amount of bleeding after cesarean section, but on the contrary, it may increase the amount of bleeding. Another strength of our study is that it is the first study in this field.
Abdominal application of fundal weight after CS has no effect on reducing post cesarean hemorrhage. Weight application does not reduce bleeding by disrupting the postpartum position of the uterus. However, weight application may be effective if devices such as pressure adjustable weight belts are developed.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
ŞP and RA designed the study and performed the research. ŞP, NY, HBK performed the research, collected data. SCO analyzied the data, helped and advised on writing manuscript. ŞP and RA wrote manuscript. MY analyzied the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Approval was obtained from the Ethics Committee of Fırat University for this prospective randomized single-center study (Ethics committee date = 15.10.2020; decision no = 2020/14-02). Written informed consent was obtained from the participants.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest. Süleyman Cemil Oğlak is serving as one of the Guest editors of this journal. We declare that Süleyman Cemil Oğlak had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Michael H. Dahan.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
