- Academic Editor
†These authors contributed equally.
Background: To investigate the diagnostic value of serum transthyretin
(TTR), placental protein 13 (PP13) and placental growth factor (PLGF) in
preeclampsia patients. Methods: Sixty cases of pregnant women with
preeclampsia who were examined in our hospital from January 2020 to February 2022
were retrospectively selected as the preeclampsia group, and 40 cases of healthy
pregnant women who received regular physical examination in our hospital during
the same period were selected as the control group. Based on the severity of the
disease, the patients were allocated into two groups: mild preeclampsia group (n
= 35) and severe preeclampsia group (n = 25). The levels of Serum TTR, PP13 and
PLGF were compared between the groups. The correlation between serum TTR, PP13,
PLGF and the patients was also analyzed by Spearman method, and receiver
operating characteristic curve (ROC) and area under the curve (AUC) was adopted
to analyze the clinical value of the separate and combined detection of serum
TTR, PP13, PLGF in the diagnosis of preeclampsia. Results: The levels of
serum TTR, PP13, PLGF in preeclampsia group were evidently lower versus the
control group (p
Preeclampsia is a major pregnancy complication after 20 weeks of pregnancy, which is manifested as elevated blood pressure, proteinuria, headache, edema and so on [1]. Preeclampsia is an important stage in the development of hypertension during pregnancy. If patients cannot be diagnosed timely and received effective intervention without delay, fetal growth restriction, oligohydramnios and other symptoms will easily appear, leading to undesirable pregnancy outcomes such as fetal growth retardation, premature labor and maternal and fetal death, which seriously threatens the life and health of mothers and infants [2]. At present, the clinical pathogenesis of preeclampsia has not been clearly elucidated. The main view is that the pathogenesis is associated with inflammatory reaction and oxidative stress, abnormal expression of cytokines, endothelial cell damage and other factors, essentially identical with the contributory factors of cardiovascular disease [3]. Ölmez et al. [4] found that the serum AQP9 concentration of pregnant women with early onset preeclampsia is significantly higher than that of normal healthy pregnant women, indicating that AQP9 may be an important biomarker for inflammatory process of early onset preeclampsia. Behram et al. [5] found that the urinary Cyr61 protein level of patients with early onset preeclampsia was higher than that of healthy pregnant women, suggesting that the increase of Cyr61 protein in the urine could be used as a prediction tool for early diagnosis of early onset preeclampsia e and renal ischemia. These findings suggest an association between abnormal trophoblast invasion and the onset of preeclampsia.
Placental protein 13 (PP13) is a galectin that is more commonly expressed in placental trophoblast cells and enters the maternal circulation through the villous space. Its abnormal expression can affect placental implantation and vascular remodeling, eventually leading to preeclampsia. PP13 has a strong immunomodulatory function, can induce the apoptosis of active T cells, and can transform and kill T lymphocytes and macrophages in the maternal decidua, so that the immune tolerance generated at the maternal-fetal interface is conducive to the maintenance of pregnancy. Abnormal immunomodulatory function of PP13 can lead to the occurrence of preeclampsia [6]. Abnormal expression of PP13 in placenta in early pregnancy is associated with the onset of preeclampsia [7]. Placental growth factor (PLGF) is a vascular endothelial growth factor that binds to the membrane surface receptor sFlt-1 and promotes the proliferation and differentiation of trophoblasts, participating in the maintenance of placental function and fetal growth and development. Its abnormal expression is related to adverse pregnancy outcomes, such as spontaneous abortion, premature birth, preeclampsia, and fetal growth restriction [8]. In the first trimester of pregnancy, impaired trophoblastic ischemia and abnormal expression of PLGF lead to poor placental vascular bed formation and abnormal uterine spiral artery remodeling in preeclampsia patients, and finally develop into preeclampsia [9]. Serum transthyretin (TTR) is a tetrameric transporter, and the serum TTR concentration in preeclampsia patients is significantly down-regulated [10]. In recent years, PP13, PLGF, and other serological markers have become the focus of clinical research on preeclampsia diagnosis [11, 12]. It has been found that the change of TTR level is related to the development of hypertension-related diseases [13]. Currently, there are few clinical reports on the diagnostic value of serum TTR, PP13 and PLGF in preeclampsia patients. For this reason, we investigated the diagnostic value in the diagnosis of preeclampsia by combined detection of serum TTR, PP13 and PLGF in order to provide a reference for clinical diagnosis and treatment of the disease.
Sixty pregnant women with preeclampsia who were examined for pregnancy in our
hospital from January 2020 to February 2022 were retrospectively selected as the
preeclampsia group. The inclusion criteria of all enrolled
patients were set in accordance with the American Association of Obstetricians
and Gynecologists (ACOG) criteria [14]. According to the specific examination
results of blood pressure and urine protein volume, the 60 pregnant women with
preeclampsia were divided into mild preeclampsia (35 cases) and severe
preeclampsia (25 cases). Inclusion criteria for the severe preeclampsia group
(any of the following conditions was met on the basis of hypertension and
proteinuria): systolic blood pressure
Preeclampsia pregnant woman inclusion criteria: (i) Those who meet the diagnostic criteria of preeclampsia in terms of clinical symptoms, signs and examination results [16]; (ii) Those without concomitant fetal growth restriction (FGR) [17]; (iii) Those without taking special drugs within 2 months before being selected as test subject; (iv) Those without obstetric complications such as premature rupture of membranes and placenta previa; (v) Those with complete clinical medical records. Exclusion criteria: (i) Pregnant women with multiple pregnancies; (ii) Those with other pregnancy complications; (iii) Patients with mental illness; (iv) Those with severe heart, liver and kidney dysfunction.
5 mL of venous blood in fasting status was taken from delivery women in the early morning of the first day after delivery. The blood samples were let stand for 1 hour, and then were centrifuged for 15 minutes with 3000 r/min. The supernatant was obtained and stored in a refrigerator for subsequent use. The levels of TTR, PP13 and PLGF were detected by immunoturbidimetry on the automatic microplate reader (Agilent Technologies, Inc., BioTek Cytation 5, Santa Clara, CA, USA), with detection wavelength of 450 nm, and the kit was manufactured by Shanghai Enzyme-linked Biotechnology Co., Ltd., (Shanghai, China) and we strictly followed the instructions to use it.
(I) Comparison of serum TTR, PP13 and PLGF levels between preeclampsia group and control group; (II) Comparison of serum TTR, PP13 and PLGF levels in patients with different severity of preeclampsia; (III) Correlation analysis between serum TTR, PP13, PLGF levels and disease progression in preeclampsia patients; (IV) ROC analysis of serum TTR, PP13 and PLGF for diagnosing preeclampsia alone or jointly.
Excel was used for statistical processing of data, and SPSS 20.0 (IBM Corp.,
Armonk, NY, USA) was used for analysis of statistical data of each index. The
count data were expressed as rate (%) and the differences between groups were
compared using chi-square test. The quantitative data were first subjected to
normality test. All included indicators were in accordance with normal
distribution, so they were expressed as mean plus or minus standard deviation
(
There were no significant differences in age, gestational weeks, and body mass
index (BMI) between the preeclampsia group and the control group in this study
(p
| Group | Number of cases | Age (years) | Gestational weeks (weeks) | BMI (kg/m |
| Control group | 40 | 28.15 |
37.68 |
28.65 |
| Preeclampsia | 60 | 27.62 |
37.52 |
27.98 |
| t | 0.485 | 0.375 | 1.355 | |
| p | 0.629 | 0.709 | 0.178 |
BMI, body mass index.
Results the levels of serum TTR, PP13, PLGF in preeclampsia group were evidently
lower versus the control group (p
| Group | Number of cases | TTR (mg/L) | PP13 (pg/mL) | PLGF (pg/mL) |
| Control group | 40 | 693.76 |
121.97 |
91.88 |
| Preeclampsia | 60 | 418.73 |
44.81 |
53.03 |
| F value | 24.608 | 17.085 | 8.936 | |
| p value |
TTR, serum transthyretin; PP13, placental protein 13; PLGF, placental growth factor.
Those of patients in mild preeclampsia group were markedly lower versus the
control group (p
| Group | Number of cases | TTR (mg/L) | PP13(pg/mL) | PLGF (pg/mL) |
| Control group | 40 | 693.76 |
121.97 |
91.88 |
| Mild preeclampsia group | 35 | 466.77 |
50.76 |
59.01 |
| Severe preeclampsia group | 25 | 364.64 |
39.89 |
49.64 |
| F value | 314.816 | 135.559 | 37.572 | |
| p value |
Notes: Comparison between mild preeclampsia group and control group, *p
TTR, serum transthyretin; PP13, placental protein 13; PLGF, placental growth
factor.
Serum TTR, PP13, PLGF levels in preeclampsia patients were negatively correlated
with the severity of disease (r = –0.332, –0.315, –0.391, p
| Indicators | Progression of preeclampsia | |
| R value | p value | |
| TTR | –0.332 | |
| PP13 | –0.315 | |
| PLGF | –0.391 | |
TTR, serum transthyretin; PP13, placental protein 13; PLGF, placental growth factor.
The area under the curve (AUC) values of TTR, PP13, and PLGF in the single diagnosis of preeclampsia
and their joint diagnosis were 0.812, 0.759, 0.867, and 0.887, respectively.
Z-test showed TTR, PP13, and combined PLGF had greater area under the ROC curve
in the diagnosis of preeclampsia than PP13 alone (p
| Indicators | 95% confidence interval | AUC | Specificity | Sensitivity |
| TTR | 0.741 |
0.812 | 0.784 | 0.721 |
| PP13 | 0.675 |
0.759 | 0.716 | 0.672 |
| PLGF | 0.805 |
0.867 | 0.797 | 0.803 |
| Joint diagnosis | 0.829 |
0.887 | 0.824 | 0.852 |
ROC, receiver operating characteristic; TTR, serum transthyretin; PP13, placental protein 13; PLGF, placental growth factor; AUC, area under the curve.
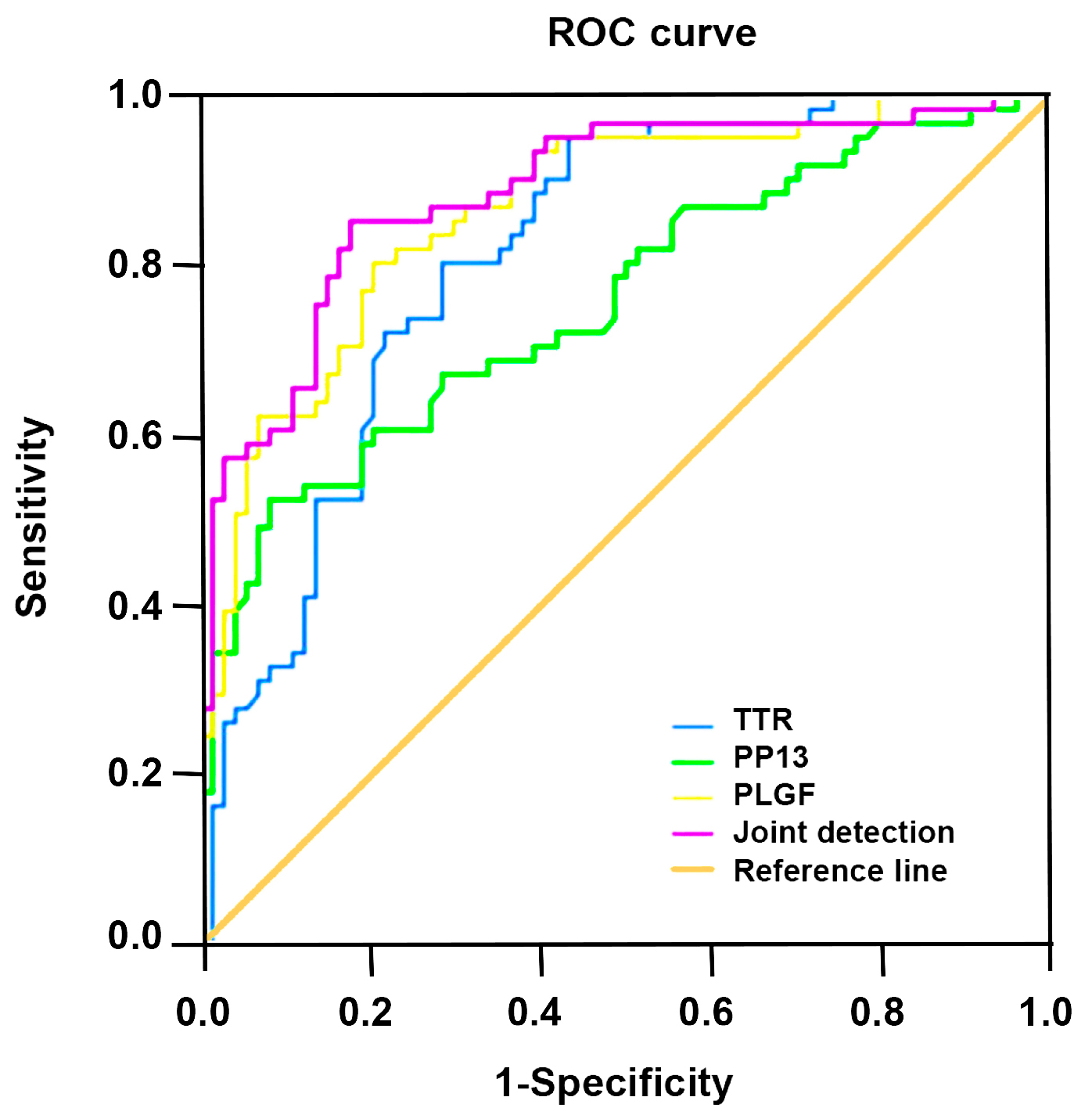 Fig. 1.
Fig. 1.Receiver operating characteristic (ROC) analysis of serum TTR, PP13 and PLGF for diagnosing preeclampsia alone or jointly.
The research on the pathogenesis of preeclampsia has always been a hot topic in obstetrics. At present, the mainstream view holds that the pathogenesis of the disease is associated with placental ischemia and hypoxia, oxidative stress, vascular endothelial dysfunction, apoptosis and so on, which is basically in line with the contributory factors of cardiovascular disease [18]. Therefore, early detection, diagnosis and treatment are of great significance in improving the outcome. It has been found that abnormal changes in vascular endothelial metabolic environment are often caused by placental arterial ischemia and hypoxia in pregnant women with preeclampsia, leading to pathological changes in placental environment and immune system and then changes in levels of various serum markers [19]. PP13 was initially isolated from the human placenta by Stepan et al. [20], it is a member of the 56 placental protein families. PP13 is a kind of galectin, which is mainly expressed in placental trophoblasts and can regulate adhesion between cells and matrix, participate in placenta implantation, maintain pregnancy, and also involves in placenta development and spiral arterioles remodeling [21]. The study confirmed that the level of PP13 in maternal peripheral blood of normal pregnant women gradually rose with increasing gestational weeks and disappeared after several weeks of delivery [22]. The results showed that serum PP13 level in preeclampsia group was markedly lower versus the control group, and that of mild preeclampsia group was obviously lower versus control group, and that of severe preeclampsia group was found to be evidently lower compared with mild preeclampsia group and control group, suggesting that the change of serum PP13 expression in pregnant women with preeclampsia may play a role in the development of preeclampsia. Furthermore, it was also found that serum PP13 level and the progression presented positive correlation. The expression of serum PP13 in preeclampsia patients decreased significantly with the progression of the disease. PP13 could be an effective predictor of preeclampsia.
Poor recasting of spiral arteries and shallow implantation of placenta occurred in pregnant women with preeclampsia due to hypertension, leading to placental hypoperfusion [23]. Placenta prowth factor (PIGF) is a member of vascular endothelial growth factor secreted by placenta, which is mostly synthesized by human umbilical vein endothelial cells and placental syncytiotrophoblasts, and it binds to the receptor via autocrine and paracrine to achieve biological functions, such as regulating the function of trophoblasts and endothelial cells, promoting placental angiogenesis, increasing vascular permeability [24]. The results showed that serum PIGF level in preeclampsia group was markedly lower versus the control group, and that of mild preeclampsia group was obviously lower versus control group, and that of severe preeclampsia group was found to be evidently lower compared with mild preeclampsia group and control group. Serum PIGF levels in patients with preeclampsia have a certain correlation with the occurrence and severity of preeclampsia. It was speculated that the underlying reason may be the impairment of trophoblast cells for some reason, which reduced PIGF synthesis and serum PIGF levels in patients with preeclampsia of pregnancy. PIGF promotes placental angiogenesis. A significant decrease in PIGF level is likely to cause placental angiogenesis and migration disorders, leading to recasting of vascular malformations, shallow embedding of the placenta, aggravation of placental ischemia and hypoxia, and the condition of pregnant women with preeclampsia, leading to the appearance of preeclampsia [25, 26].
TTR, also known as prealbumin, is an important factor transporting retinol-binding protein and thyroid hormones, which is mostly synthesized in the liver, but also in a small amount in placenta, yolk sac, small intestine, retina, meninges and other tissues [27]. TTR can selectively deposit under small arterial intramembrane by means of denaturation, resulting in stenosis of the arterial lumen and ultimately ischemia and hypoxia in the perfusion tissue [28]. It was found that TTR was under-expressed in non-pregnant women, but peaked in mid-pregnancy and remained low in late pregnancy as pregnancy progressed [29]. The results showed that serum TTR level in preeclampsia group was markedly lower versus the control group, and that of mild preeclampsia group was obviously lower versus control group, and that of severe preeclampsia group was found to be evidently lower compared with mild preeclampsia group and control group. The study found that different oxidizing reactions in pregnant women with preeclampsia could produce amyloidosis in TTR and deposit in placental tissue in clustering method, having a toxic effect on placental tissue and aggravating placental dysfunction, and in return affecting the placental secretion of TTR and further reducing the expression of serum TTR [30]. In addition, the selective deposition of TTR in the maternal arteriolar intima could lead to narrowing of the lumen and tissue ischemia, thereby worsening placental ischemia and hypoxia in preeclampsia and the condition of the patients [31, 32]. The AUC values of TTR, PP13, PLGF in the diagnosis of preeclampsia, alone or jointly, were 0.812, 0.759, 0.867 and 0.887. Combined detection of serum TTR, PP13 and PLGF in the diagnosis of preeclampsia is of high clinical value.
In this study, preeclampsia patients with various degrees of severity were enrolled as the research object, and the diagnostic value of serum TTR, PP13, and PLGF joint detection in preeclampsia patients was explored. The results showed that serum TTR, PP13, and PLGF levels in preeclampsia patients were significantly lower than the control group, and with the aggravation of the disease the serum TTR, PP13, and PLGF levels were lower, suggesting that serum TTR, PP13, and PLGF levels were closely related to the occurrence and development of preeclampsia. The joint detection of serum TTR, PP13, and PLGF had high clinical value in the diagnosis of preeclampsia. However, there were still some shortcomings in this study. This study was a retrospective study, and the included sample size was limited. In the future work, the sample size was further expanded to analyze the specificity and sensitivity of serum TTR, PP13, and PLGF levels in the diagnosis of preeclampsia with different severity levels. In conclusion, this study has provided a certain clinical basis for the diagnosis of preeclampsia.
The datasets supporting the conclusions of this article are included within the article.
YJ—designed the research study, performed the research and drafting of the manuscript; CFD—analysis of data and manuscript revision; XHC and XFC—design, revision and statistical analysis. All authors contributed to editorial changes in the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
The study was approved by the Ethics Committee of our hospital (code number: HCMC241) and all study subjects signed the informed consent form.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
