- Academic Editor
†These authors contributed equally.
Background: Pathophysiology of placental syndromes is still unclear, and umbilical cord-derived mesenchymal stem cells (UC-MSCs) might play a role in the development of these syndromes. In this prospective cohort study, we evaluated proliferative abilities of two types of UC-MSCs, Wharton’s Jelly MSCs (WJ-MSCs) and cord blood MSCs (CB-MSCs), in placental syndromes. Methods: A total of 16 cord blood and umbilical cord samples were seeded and cultured until MSC growth potential exhaustion. Cumulative population doublings were employed for studying growth potential, and flow cytometry immunophenotyping for verification of mesenchymal markers. Results: In our prospective cohort study, on one hand CB-MSCs from pathological pregnancies showed a significant reduction of growth potential, on the other hand WJ-MSCs showed a trend toward higher growth potential. This trend is consistent with the well-known faster-growing phenotype of WJ-MSCs under low oxygen atmosphere. Moreover, it’s well understood that chronic hypoxia is a main feature of both intrauterine growth restriction (IUGR) and preeclampsia, thus, our data perfectly match with the well-known clinical characteristics. Conclusions: Growth potential of CB-MSCs obtained from placental syndromes tended to be reduced compared to that of MSCs from healthy pregnancies. Our results need to be confirmed in larger in vitro studies, as a higher number of CB- and WJ-MSC would better clarify pathophysiology of placental syndromes.
Inadequate placental development and subsequent inappropriate mother-fetus exchanges are responsible for preeclampsia and intrauterine growth restriction (IUGR) linked to elevated maternal and fetal morbidity and mortality, recently defined as “placental syndromes” [1]. The exact pathophysiology behind these syndromes is still unclear, however, an incomplete uterine spiral artery remodeling with the lack of uteroplacental vessel development is one of the driver events [1, 2, 3, 4]. Physiologically, uteroplacental vessels have the function of showing down arterial pressure, lowering vessel resistance and increased the flow at the placental level [2]. Therefore, the absence of this system causes tissue damage, preeclampsia, IUGR, and umbilical cord flow changes detected by doppler ultrasound are typically found in placental syndromes [2, 3, 4, 5].
Wharton’s Jelly mesenchymal stem cells (WJ-MSCs) are stem cells with high
differentiative potential, which also play a role in placental syndrome
development. WJ-MSCs might act as adventitia for umbilical vessels and might
differentially express extracellular components such as growth factors during
preeclampsia, oligohydramnios, IUGR, low birth weight, or the life-threating
condition known as “lean umbilical cord” with birth growth restriction and
distress [6, 7]. Mesenchymal stem cells with high proliferative and
differentiation potentials can be isolated from umbilical cord blood (cord blood
mesenchymal stem cells (CB-MSCs)). Collectively, WJ- and CB-MSCs are termed
umbilical cord-derived mesenchymal stem cells (UC-MSCs), a class of extra-fetal stem
cells present in the placenta, amniotic lining, and amniotic fluid [8, 9, 10].
UC-MSCs deeply interact with component of the maternal-fetal interface, as
WJ-MSCs cultured with trophoblast cells promote trophoblastic proliferation,
migration, and invasion, and modification in the expression of human chorionic
gonadotropin, placental growth factor, and endoglin [11, 12, 13]. Moreover, CB-MSC
infusion in rats with preeclampsia improves weight at birth, improves uterine
spiral arteries remodeling and renal function, and reduces tumor necrosis factor
(TNF)-
Based on this evidence, we evaluated proliferative potentials of UC-MSCs from pregnant women with placental syndromes to better understand the pathophysiology behind these maternal-fetal disorders.
A total of 38 pregnant women were considered eligible for this prospective cohort study conducted at the University Hospital “San Giovanni di Dio e Ruggi d’Aragona”, Salerno, Italy, and at the Hospital “San Giuseppe Moscati”, Avellino, Italy, and 35 of them were enrolled (Table 1). Study protocol and written informed consent were reviewed and approved by local Institutional Review Board (Ethics Committee “Campania Sud”, Brusciano, Naples, Italy; prot./SCCE n. 24988). Subjects have given their written informed consent before sampling. 10 of them were diagnosed with placental syndromes: 6 with preeclampsia and 4 with IUGR. 28 patients had physiological pregnancies. Inclusion criteria for the pathologic group were an antepartum diagnosis of preeclampsia or IUGR.
| Pathological group (n = 10) | Physiological control group (n = 25) | p value | ||
| Maternal age (years) | 32.78 | 32.77 | ||
| Mean gestational age (weeks) | 37.86 | 38.96 | ||
| Mean birthweight (g) | 2743.18 | 3058.95 | ||
| Delivery route (%) | ||||
| Cesarean section | 92.3 | 82.6 | ||
| Vaginal route | 7.7 | 17.4 | ||
A p value
Human WJ-MSCs were isolated from fresh human umbilical cords placed in 0.9%
NaCl physiological solution supplemented with 1 g of ampicillin (Sigma Aldrich,
Milan, Italy) and 500 mg of sulbactam (Sigma Aldrich, Milan, Italy), stored at 4
°C, and processed within 4 h after collection. Briefly, samples were cut
in small segments, washed in fresh medium to remove blood and debris, cut open
lengthwise with sterile scissors, and arteries and veins were removed. Each piece
was then transferred to a 175 cm
After vaginal or caesarean delivery, umbilical cord was clamped and disinfected;
and 35 mL of blood was collected in sterile BD Vacutainer Z (no Additive)
collection tubes (BD Diagnostic, Oxford, UK) containing 1 mL of citrate-phosphate
dextrose as anticoagulant (MacoPharma, Tourcoing, France). Fresh whole blood was
centrifuged for 15 minutes at 1900 rpm at 20 °C, and then the buffy coat
interphase layer was collected, placed in a 15 mL falcon tube (BD Biosciences,
Oxford, UK), and mixed with an equal volume of 0.9% NaCl physiological
solution, and mononuclear cells were isolated by Ficoll-Paque density gradient
centrifugation as per manufacturer’s instructions. Cells were then seeded at a
density of 5
Flow Cytometry immunophenotype of WJ- and CB-MSCs was performed on cells at first culture passage. Briefly, samples were incubated with the following mouse anti-human antibodies: cluster of differentiation (CD)90-fluorescein (FITC), CD45-Phycoerythrin (PE)-Cyanine7 (PC7), CD105-PE, CD56-PE-Cyanine5 (PC5), CD14-PC7, CD45-PC7, human leukocyte antigen (HLA)-DR-FITC, CD34-PE (Beckman Coulter, BC, Fullerton, CA, USA), and CD73-APC (Miltenyi Biotec, Gladbach, Germany), as described elsewhere [15]. Sample acquisition was carried out using a BD FACSVerse flow cytometer (BD Biosciences) equipped with blue (488 nm) and red lasers (628 nm) and BD FACSuite software (BD Biosciences, Oxford, UK). PMT voltages setting, and compensation were performed using single-color controls for each fluorochrome and an unstained sample as negative control. All samples were run with the same PMT voltages, and a minimum of 10,000 events were recorded.
Population doubling was calculated for WJ-MSCs and CB-MSCs using the following
equation: population doubling (PD) = log
Data were analyzed using SPSS software (v.2.0; SPSS Inc., Chicago, IL, USA) and Prism (v.9.3.1; GraphPad Software, San Diego, CA, USA).
A power calculation was performed to determine an appropriate sample size for this noninferiority study. We set the mean population doubling numbers at 10. The standard deviation was set as 1. This power calculation indicated that 32 patients in each group would be necessary to detect a 15% difference in with a power of 80% at a 0.5% level of significance.
For flow cytometry data, results are presented as percentage of positive cells,
and expression of each marker on single cells is also reported as histograms and
using unstained samples as negative controls. For characterization of mesenchymal
cells, percentage of positive cells. Shapiro-Wilk’s test for parametrical
distribution and t-test were employed for statistical comparisons
between groups. A p
From the 35 patients enrolled in this prospective cohort study, a total of 28 umbilical cord samples and 31 cord blood samples were obtained. Of the 28 umbilical cord blood samples, 11 resulted in WJ-MSC growth: (i) 10 from physiological pregnancies; (ii) 1 from pathological pregnancy, specifically, from IUGR. Of the 31 collected cord blood samples, 5 resulted in CB-MSC growth: (i) 3 from physiological pregnancies; (ii) 2 from pathological pregnancy, specifically, 1 from a preeclampsia and 1 from IUGR. The overall growth percentage of CB- and WJ-MSCs was 16.1% and 39.2%, respectively. Percentage of cord blood and umbilical cord samples that failed in cellular growth for any reason was compared to that of samples that resulted in MSC colonies by Chi square test. Umbilical cord samples tended to have increased ability to result in MSC colonies compared to cord blood.
Next, cellular growth rates of MSCs obtained from cord blood or umbilical cord
samples were analyzed. Of the 5 cord blood samples that originated o MSC
colonies, CB-MSCs from pathological placenta showed a slower growth rate compared
to those obtained from physiological pregnancies (Fig. 1 and Table 2). Moreover,
CB-MSCs from pathological samples, ultimately resulted in cell death. Conversely,
WJ-MSCs from the pathological group showed faster growth rate compared to that
observed in the physiological group (Fig. 2 and Table 2). To confirm our result,
we divided physiological pregnancies into two groups based on the newborn’s
weight (
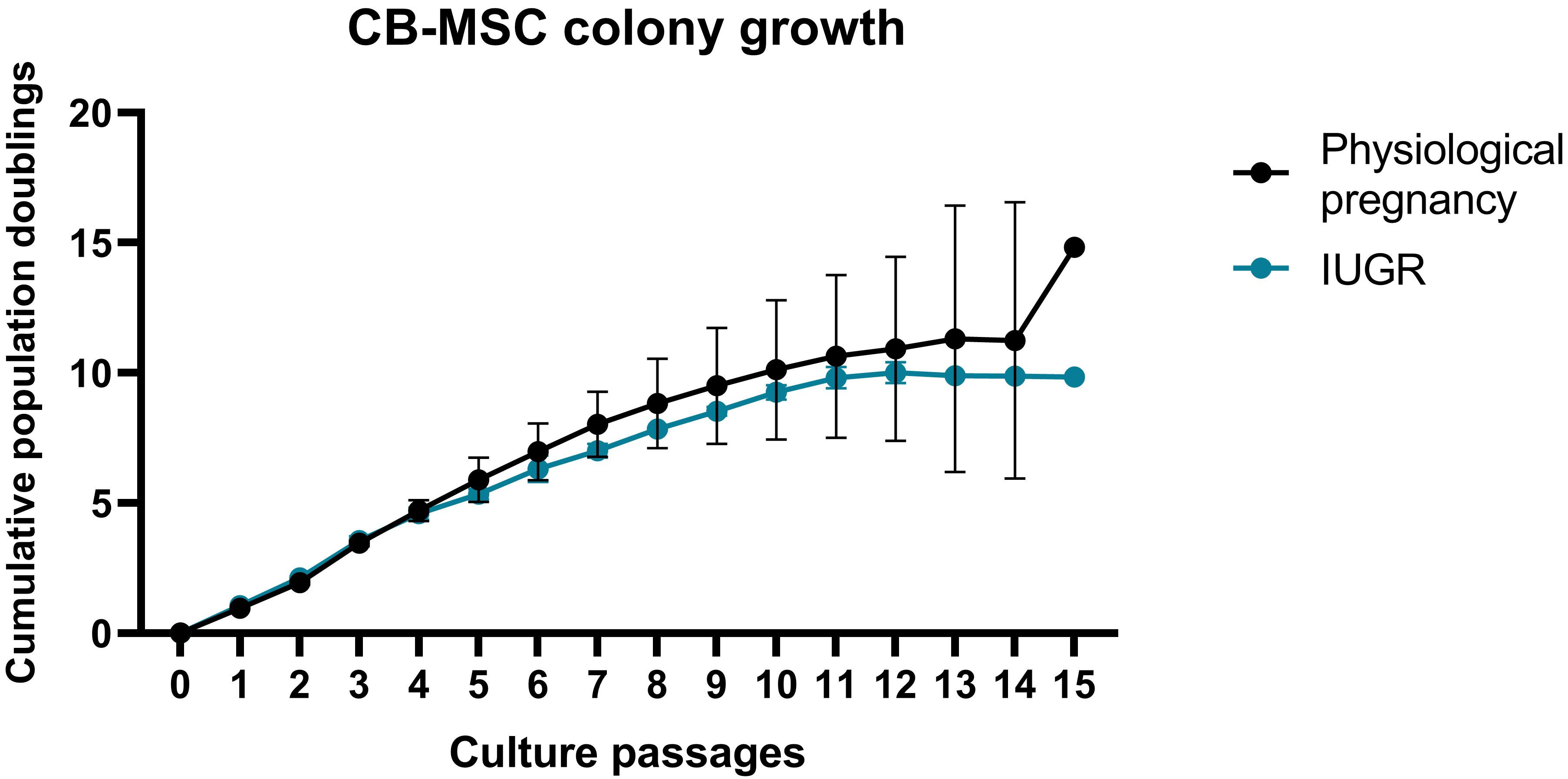 Fig. 1.
Fig. 1.Cord blood derived mesenchymal stem cell (CB-MSC) growth curves. Each CB-MSC growth curve from every healthy pregnancy is reported as shades of blue curves, while each CB-MSC growth curve for each pathological pregnancy is shown as shades of red curves. IUGR, intrauterine growth restriction.
| Physiological vs. Pathological | CB-MSCs (n = 5, 3 physiological vs. 2 pathological) | WJ-MSCs (n = 11, 10 physiological vs. 1 pathological) |
| Mean |
0.54 |
1.09 |
| SD mean error | 0.137 | 0.275 |
| 95% CI | 0.2407 to 0.8449 | 0.5101 to 1.6826 |
| T | 3.954 | 3.986 |
| p value | 0.002 | 0.001 |
A p value
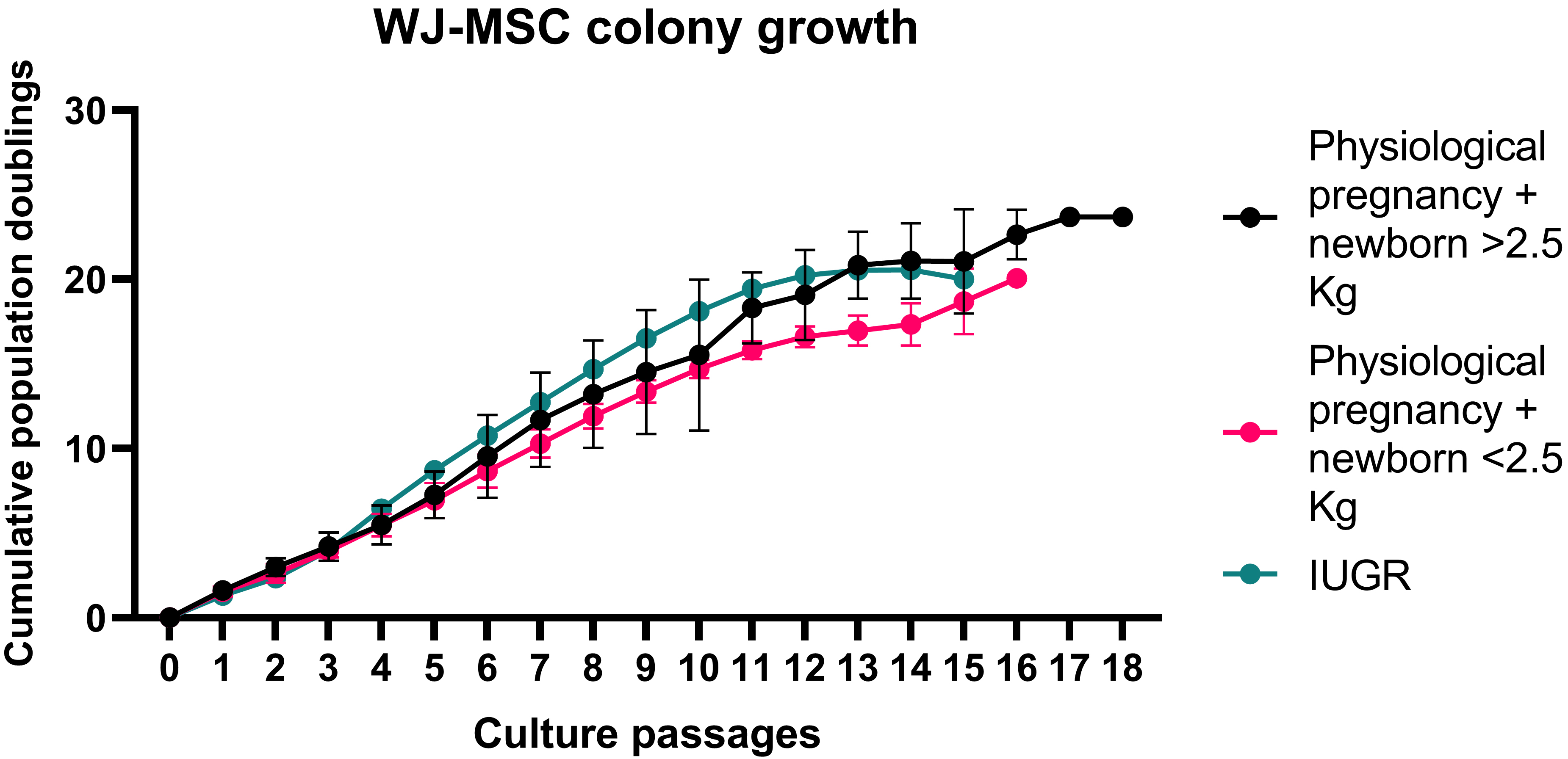 Fig. 2.
Fig. 2.Wharton’s Jelly mesenchymal stem cell (WJ-MSC) growth curves. Each WJ-MSC growth curve from every healthy pregnancy is reported as shades of blue curves, while each CB-MSC growth curve for each pathological pregnancy is shown as shades of red curves. IUGR, intrauterine growth restriction.
Finally, immunophenotype of CB- and WJ-MSCs was evaluated by flow cytometry (Fig. 3). Of the 5 cord blood samples that originated MSC colonies, flow cytometry immunophenotype was performed on all three CB-MSCs from healthy pregnancies, while no pathological samples were analyzed due to premature death of cultured cells. Of the 11 UC-MSCs, flow cytometry analysis was not performed on one sample of the control group and one of the pathological groups, because of earlier cell death that did not allow further immunophenotype analysis. Both CB- and WJ-MSCs showed a mesenchymal phenotype with positivity for CD90, CD73, and CD105, and negativity for CD45, HLA-DR, CD34, CD14, and CD56, and no differences were described between the two sources of stem cells and between healthy and pathological pregnancies.
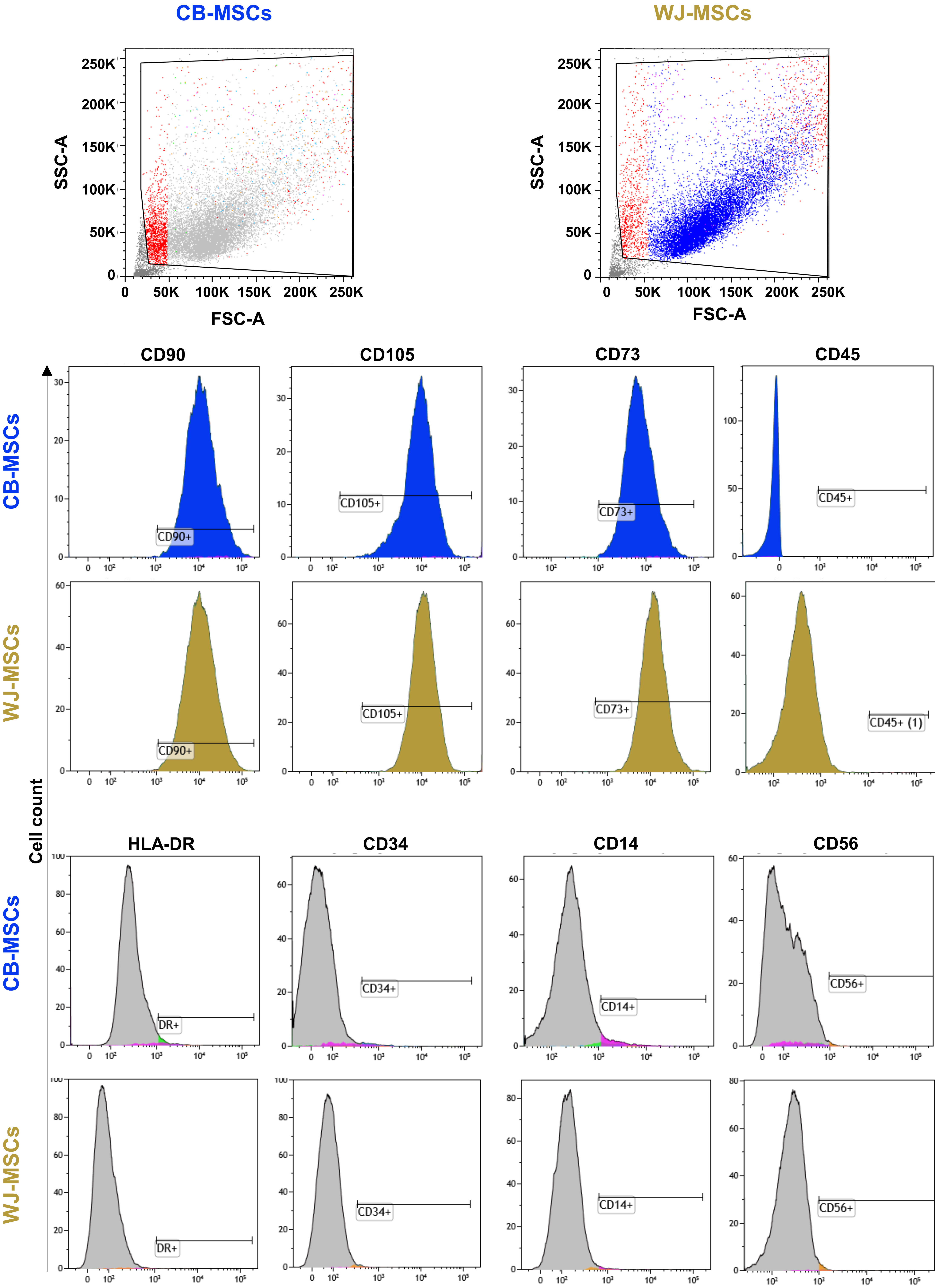 Fig. 3.
Fig. 3.Cord blood derived (CB-MSCs) and Wharton’s Jelly-derived mesenchymal stem cell (WJ-MSCs) immunophenotype. Flow cytometry analysis was performed on CB- and WJ-MSCs, and cells were positive for mesenchymal markers, such as CD90, CD73, and CD105, and negative for lineage-specific markers, such as HLA-DR, CD45, CD34, CD14, and CD56. SSC-A, side scatter area; FSC-A, forward side scatter-area; CD, cluster of differentiation.
In this prospective cohort study, differences in growth potential between cord blood and umbilical cord derived-MSCs from healthy and placental syndromes affected pregnancies, such as preeclampsia and IUGR, were evaluated. Reported rates of CB-MSCs isolation range from 1% to 25%; therefore, our CB-MSCs isolation rate of 16.1% is consistent with that described in literature [14, 15, 16]. Conversely, our rate of WJ-MSC isolation of 39.2% was significantly lower than that reported in literature, which was described to be close to 100% [17]. This difference could be related to umbilical cord conditions and placental integrity that are higher for caesarean deliveries [18], while they could decrease in samples obtained from vaginal deliveries, as described in our study. Indeed, preeclampsia and IUGR are not an absolute indication for caesarean section (C-section) delivery that is chosen based on obstetrics situation and disease severity [5]. Therefore, sample collection only from C-section would have resulted in a bias, excluding mild cases from our study.
Growth potential of CB-MSCs obtained from placental syndromes were reduced
compared to that of MSCs from healthy pregnancies. However, no differences in
immunophenotyping were described, as previously reported [19]. Previous studies
have documented a reduced proliferative capacity of placental MSCs obtained from
pregnancies complicated by IUGR and preeclampsia [20, 21]. This reduced potential
might be related to impairment in number and proliferation capacity of
endothelial cell progenitors, hematopoietic stem cells, and endothelial colony
forming cells in umbilical cord from pregnancies complicated by placental
syndromes [17, 22, 23]. Indeed, CB-MSCs infusion improves clinical outcomes of
preeclampsia in rat models by reducing proteinuria, blood pressure,
proinflammatory cytokine levels (e.g., TNF-
On the other hand, WJ-MSCs enhance trophoblasts in vitro migration and
invasion capacities [19]. Therefore, highest proliferation capacity of WJ-MSCs is
consistent with literature, stating that WJ-MSC has a faster growth potential
under low oxygen tension [24, 25, 26, 27]. These behaviors of both CB- and WJ-MSCs might
be implicated in the development of placental syndromes, as documented in our
study, showing decreased growth potential of CB-MSCs obtained from pregnancies
complicated by IUGR and preeclampsia. Indeed, chronic hypoxia could be the
missing factor of why preeclampsia and IUGR set on and/or develop, negatively
impairing the known favorable effects of UC-MSCs on placentation [28, 29, 30]. This
finding could be supported by the lower growth rate observed in those
physiological pregnancies who delivered SGA newborns. Indeed, we hypothesize
that, as SGA newborns are not affected with chronic hypoxia as IUGR newborns,
their WJ-MSCs growth is not influenced by low tension O
Limitations of our study are: (i) the small number of samples; (ii) lack of more in-depth analysis, such apoptosis rate by flow cytometry or differentiation potential to other lineage of obtained MSCs; and (iii) confirmation of reduced proliferation capacity of CB-MSCs by comparison of early and late passages.
Our results open new scenarios on possible roles of UC-MSCs in placental development also suggesting these stem cells sources as potential therapeutic strategy for IUGR and preeclampsia; however, further studies are required to confirm our hypothesis.
The data that support the findings of this study are available on request from the corresponding author.
Conceptualization: LM, MAC and BS; methodology: LM, SGC, PM, FP, MRC, CS, and VG; clinical patients’ sample and data collection: MAC, CF, MP, and MG; formal analysis: CF, PM, and VG; writing—original draft preparation: LM, MAC, and VG; writing—review and editing: CS and BS. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Study protocol were reviewed and approved by local Institutional Review Board (Ethics Committee “Campania Sud”, Brusciano, Naples, Italy; prot./SCCE n. 24988). Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Not applicable.
This research was supported by the Intramural Program of the Department of Medicine, Surgery and Dentistry, University of Salerno, Italy.
The authors declare no conflict of interest. Maria Antonietta Castaldi is serving as one of the Guest editors of this journal. We declare that Maria Antonietta Castaldi had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Michael H. Dahan.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
