- Academic Editor
†These authors contributed equally.
Background: Prolonged pretreatment time may be harmful to frozen embryo’s developmental potential. This study was conducted to evaluate the effect of different equilibration times on the clinical and neonatal outcomes of frozen-warmed blastocyst transfer. Methods: This is a retrospective study based on data collected from our medical records from March 2018 to March 2022 and including a total of 763 expanded blastocysts from 538 warming blastocyst cycles. These cycles were divided into two groups according to the equilibration time: (A) 6–7 minutes, and (B) 9–10 minutes. The survival rate, clinical, and neonatal outcomes were investigated. Results: The survival, implantation, and clinical pregnancy rates of vitrified-warmed shrinkage blastocyst were not different between the two groups. Other variables analyzed including live birth, multiple gestation, and neonatal outcomes were similar between the two groups. Conclusions: The results of this study illustrated that vitrification of artificially collapsed blastocysts with a shorter equilibration time (6–7 minutes) and pre-vitrification is able to lead to similar clinical and neonatal outcomes in patients undergoing assisted reproductive technology (ART).
Since the birth of Louise Joy Brown in 1978, assisted reproductive technology (ART) has been constantly increasing, and have permitted millions of infertile couples to conceive. Currently, more than 3.5 million cycles are annually performed, with over 500,000 deliveries worldwide. It has been estimated that about 9 million children have been conceived globally following ART [1]. The advancements in ART have been achieved thanks to several improvements, including ovarian stimulation procedures, fertilization, embryo transfer methods, and importantly to embryo cryopreservation. Vitrification has become a highly important step of ART for a variety of reasons: to store supernumerary embryos for future use, for preimplantation genetic testing, or freeze-all cycle, once the patient is exposed at high risk to develop ovarian hyperstimulation syndrome (OHSS) and the fresh transfer cannot be performed [2, 3]. Cryopreservation of oocytes and embryos by “slow freezing” was first applied in the 1980s [4], which was subsequently replaced by the “vitrification” procedures [5]. This practice has been considered a real breakthrough in ART, especially with oocyte cryopreservation, allowing embryologists to obtain a higher survival rate at the warming, fertilization rates, and embryonic development compared to the slow freezing procedure [6, 7]. Practically, cryopreservation enables long-term preservation of cells (gametes/embryos) at ultra-low temperatures in a state of suspended animation. To obtain that state, it is fundamental to avoid ice crystals formation, which will damage irreversibly the cell and induce death. This can be achieved through vitrification, using a high concentration of cryoprotective agents (CPAs) to increase viscosity, inhibit the growth and formation of ice crystals, and finally induce the solution to enter a “glassy state” [8]. The ability to cryopreserve human embryos has also improved significantly in the last decade [6, 9, 10]. There is some sufficient evidence showing results from vitrification are superior to those achieved with the slow freezing protocols [11, 12, 13, 14, 15, 16]. However, the methods utilized to cryopreserve human embryos still have some weak points that might be improved. Several approaches have been applied and tried to optimize the vitrification procedure. These procedures employed are not always designed to specifically take account of the osmotic tolerance response of the cells according to the temperature, time, and exposure to CPAs [17]. Thus, a critical aspect is represented by the high concentration of CPAs. One of the most used for cryopreserved gametes and embryos is dimethyl sulfoxide (DMSO), an amphipathic chemical compound. Exposure to this molecule might cause unexpected changes in cell fates, probably affecting epigenetic regulation, especially when used at high concentrations [18, 19]. Further, CPA might impact negatively cellular metabolism and function, enzyme activities, cell growth, and apoptosis [20], and might be correlated with increased levels of reactive oxygen species (ROS) and apoptotic events [21]. Several publications reported that human embryos should be kept in the vitrification solution for a maximum of 1 minute [12, 13, 14], while the equilibration times normally ranged from 5 to 15 minutes [15, 16, 17, 18]. The exposure time of embryos to CPAs represents an important concern for the success of vitrification. Longer exposure to equilibration solutions may be detrimental to further embryo development, while a briefer time may not be enough for the penetration of CPAs into the cells. Contrasting results have been found in the literature, with studies reporting a fixed equilibration time of 5 minutes and others increasing the exposure time up to 15 minutes [22, 23, 24, 25, 26, 27, 28]. These variations imply that an agreement is missing on the equilibration time for human embryos vitrification and, therefore, the need to improve our vitrification protocol. We retrospectively analyzed our results to investigate the effects of different equilibration times on embryo survival, clinical, and neonatal outcomes.
This was a retrospective cohort study performed at Center for Reproductive
Medicine of Mary Hospital, from March 2018 to March 2022, and included 538 frozen
embryo replacements with women aged
Ovarian stimulation and blastocyst preparations were achieved using the protocol
as described in our previously reported method [11, 12, 13]. Patients were treated
with either a long gonadotropin-releasing hormone (GnRH) agonist (triptorelin,
Diphereline, Ipsen, France) or a GnRH antagonist (cetrorelix, Cetrotide, Merck
Serono, Switzerland) protocol. Ovulation trigger was achieved with recombinant
human chorionic gonadotropin (rhCG, Ovidrel, MerckSerono) as soon as 50% of the
follicles of
COC were isolated from follicular fluid, rinsed, and transferred to 0.6 mL of
Universal in vitro fertilization (IVF) Medium (CooperSurgical Fertility Solutions, Malov, Denmark),
covered with oil for culture tissue (CooperSurgical Fertility Solutions, Malov,
Denmark) in four-well dishes (Nunc™, ThermoFisher Scientific, Mexico city,
Mexico), and returned to the incubator (Astec Co., Ltd, Fukuoka, Japan)
equilibrated at 37 °C, 6% CO
The morphologic features of the blastocysts were assessed on day 5 according to Gardner’s score [29]. The best quality blastocyst was replaced in embryo transfer medium; any remaining good quality blastocysts were cryopreserved. Some patients had no fresh embryo replacement, and all the blastocysts were vitrified for future use. Most of the blastocysts were cryopreserved on day 5 (93.1%), the remaining on day 6.
Hatching and expanded blastocysts (grade 3 or more) were artificially shrunk by applying one or two laser pulses (Hamilton Thorn Bioscience Inc, Beverly, MA, USA) before vitrification. The blastocyst was positioned to provide a safe distance between the inner cell mass (ICM) and the focus of the laser beam before being exposed to a minimum setting (200 ms) laser pulse to produce a small hole at the junction of two trophectoderm cells, resulting in the discharge of fluid from the blastocoel cavity (Fig. 1A). Normally, a blastocyst shrinkage occurred within 1 or 2 minutes. Rarely a second laser pulse was applied, and for some blastocysts responding slowly, it took up to 5–8 minutes to observe the shrinkage and disappearance of the blastocoel (Fig. 1B). Subsequently, the embryo was rapidly vitrified.
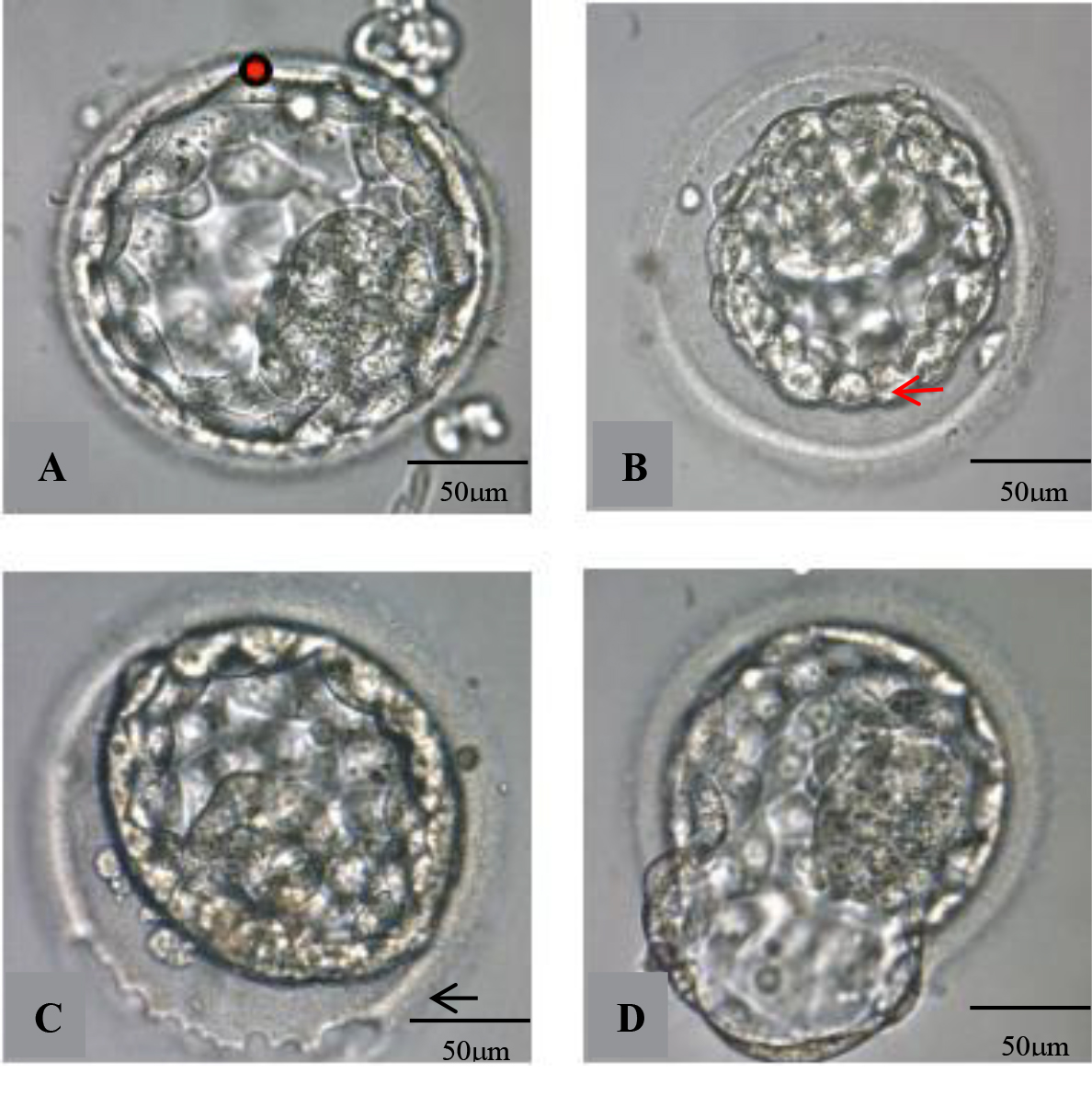 Fig. 1.
Fig. 1.Photographs of AS and laser-assisted hatching of blastocysts.
(A) Laser drilling at the cellular junction of the trophectoderm (red arrow)
before vitrification (red dot). (B) Blastocyst after AS. (C) A continuous laser
beam was emitted tracing the zona pellucida (ZP) (black arrow) to drill a hole in
about one fourth of the ZP surface. (D) Blastocyst partly hatched from ZP after
about two hours’ culture. AS, artificial shrinkage; ZP, zona pellucida.
Magnification is 400
The Cryotop® method (Kitazato Cryotop®, Kitazato Corporation, Shizuoka, Japan) initially described by Kuwayama and colleagues [5, 32, 33] was applied for blastocyst vitrification. The procedure comprised two different steps: equilibration and vitrification, which are both carried out at room temperature (22–25 °C). Following the shrinkage, the blastocyst was transferred into the equilibration solution (ES) for 6–7 minutes (group A) or 9–10 minutes (group B). In the second phase, the embryo was exposed to a vitrification solution (VS) containing dimethyl sulfoxide (DMSO) in combination with ethylene glycol, trehalose, which functions as an osmotic agent, gentamicin, and hydroxypropyl cellulose for 45–60 seconds. Blastocysts were immediately placed on the Cryotop® device, using a narrow and sterile micropipette (Kitazato Biopharma Co. Ltd., Fuji, Shizuoka, Japan), with the smallest possible amount of VS, and were quickly immersed into liquid nitrogen (LN2). A single blastocyst was always vitrified on each Cryotop® device.
The Cryotop® Thawing Media Kit (Kitazato
Cryotop®, Kitazato Corporation, Shizuoka, Japan) was used for
warming. In a Nunc 35
After two hours of culture, the embryo was reassessed and often the re-expansion of the blastocoel was observed (Fig. 1D). Embryo transfer was normally performed within 2 to 3 hours. Patients received one to a maximum of two blastocysts, based on quality. In case the embryo did not survive, another available embryo was warmed; otherwise, the transfer was cancelled. All frozen-warmed embryo cycles were transferred in day 5 to the endometrium.
Common modalities for blastocyst transfer were natural cycles or hormonal
replacement cycles for endometrial preparation. In women with
regular menstrual cycles, warmed blastocysts were transferred into the uterus
during natural cycles that were monitored with ultrasound and in which ovulation
was confirmed based on urine luteinizing hormone (LH) tests
(Tianjin Recare Co., Ltd., Tianjin, China). For artificial preparation of
endometrium, the administration of progesterone (50 mg in oil, daily) (cetrorelix, Cetrotide, Merck Serono, Switzerland) was
initiated when endometrial thickness exceeded 8 mm. On day 5 after the initiation
of progesterone treatment, the blastocysts were warmed and the surviving
blastocysts were transferred into the patient’s uterus using embryo transfer
catheters (Cook Incorporated, Bloomington, IN, USA) under ultrasound guidance (Kaixin, Xuzhou, Jiangsu, China)
[31, 34]. For luteal supplementation, progesterone pessary was utilized, which was
continued daily for at least 2 weeks after embryo transfer.
Clinical outcomes in this study included: implantation rate (IR), clinical
pregnancy rate (CPR), live birth rate (LBR), miscarriage rate, and multiple
pregnancy rate (MPR). IR was confirmed when a gestational sac was visualized via
an ultrasound examination. CPR was defined as the detection of a foetal
heartbeat. LBR was calculated by dividing the number of live birth deliveries by
the number of transfers performed. The loss of a foetus with a gestational age of
Data were presented as mean
A total of 763 vitrified-warmed blastocysts were analyzed in this study, of
which 758 survived at the warming step (99.3%; 758/763). All surviving
blastocysts were replaced in 538 women. The patient’s characteristics are
depicted in Table 1. No significant differences were observed regarding the mean
age of patients (30.17
| Group A | Group B | p-value | ||
| Transfer cycles | 250 | 288 | ||
| Maternal age (years) | 30.17 |
29.50 |
0.767 | |
| Transferred blastocyst (n) | 1.50 |
1.33 |
0.599 | |
| Basal FSH (mIU/mL) | 7.33 |
7.00 |
0.734 | |
| Maternal BMI | 22.67 |
21.17 |
0.254 | |
| Infertility duration (years) | 4.17 |
4.67 |
0.604 | |
| Primary infertility (%) | 65 (26.00) | 62 (21.5) | 0.223 | |
| Endometrial thickness (mm) | 8.83 |
9.0 |
0.828 | |
| Artificial cycle, n (%) | 215 (86.00) | 232 (80.6) | 0.093 | |
| Natural cycle, n (%) | 35 (14.0) | 56 (19.4) | ||
Data are presented as mean
FSH, follicle-stimulating hormone; BMI, body mass index; SD, standard deviation.
Regarding clinical outcomes (Table 2), results show a similar survival rate after warming for group A (99.4%) and group B (99.3%), as well as the same implantation rate (A: 59.1% vs B: 61.2%), and clinical pregnancy rate (A: 70.4% vs B: 68.4%) for both groups. Further, the live birth (A: 64% vs B: 57.3%), multiple gestation rates (A: 21.0% vs B: 24.4%), and spontaneous miscarriage rate (A: 9.1% vs B: 14.2%) were comparable in the two groups.
| Group A | Group B | p-value | |
| Survived blastocysts (%) | 99.4 (359/361) | 99.3 (399/402) | 1.000 |
| Implantation (%) | 59.1 (212/359) | 61.2 (244/399) | 0.555 |
| Clinical pregnancy (%) | 70.4 (176/250) | 68.4 (197/288) | 0.616 |
| Live birth (%) | 64 (160/250) | 57.3 (165/288) | 0.113 |
| Spontaneous miscarriage (%) | 9.1 (16/176) | 14.2 (28/197) | 0.126 |
| Multiple gestation (%) | 21.0 (37/176) | 24.4 (48/197) | 0.442 |
Data are presented as proportion (%).
Table 3 depicts the neonatal outcome of patients who completed the
vitrified-warming program. In group A, a total of 180 children were born,
vs group B, 199 children were born. There were no differences between
the two groups concerning the prevalence of male babies (A: 54.4% vs B:
54.3%), average gestational age (A: 38.67
| Group A | Group B | p-value | |
| Male babies (%) | 54.4 (98/180) | 54.3 (108/199) | 0.973 |
| Gestational age (weeks) | 38.67 |
38.33 |
0.664 |
| Preterm birth (%) | 20 (36/180) | 20.6 (41/199) | 0.884 |
| Birth weight (kg) | 2.95 |
3.05 |
0.780 |
| Low birth weight (%) | 21.1 (38/180) | 25.1 (50/199) | 0.355 |
| Macrosomia (%) | 3.3 (6/180) | 2.0 (4/199) | 0.630 |
| Cesarean section (%) | 65.6 (105/160) | 68.5 (113/165) | 0.583 |
| Congenital abnormalities (%) | 1 | 0 |
Data are presented as mean
The objective of this study was to investigate the effects of
different equilibration times on clinical and neonatal outcomes
of human blastocysts vitrified following AS
with the laser pulse. Results demonstrate that a shorter equilibration time of
6–7 minutes resulted in optimal survival, clinical pregnancy, and live birth
rates, suggesting that the extension of ES to 9–10 minutes does not bring any
further benefits to the vitrification process. In ART, a two-step protocol is
commonly applied to vitrify human embryos, and in China, this protocol is very
popular and adopted by several IVF units. Embryos in the ES are in contact with a
lower concentration of CPAs and rapidly start losing water. Lately, in the VS,
embryos are exposed to a higher percentage of CPAs, which induces a profound
volumetric change and osmotic imbalance of the embryos. Exposure to high
concentrations of CPAs is thought to be very critical for the efficiency of
vitrification [35], considering that high CPAs might cause cytotoxicity, osmotic
stress, and epigenome alterations [36, 37]. Thus, extended exposure to ES might
be detrimental and impair future embryo development, while a shorter exposure may
not be enough for the penetration of CPAs into the cells, therefore the balance
between CPAs concentration and time of embryo exposure is decisive for
vitrification success [17, 19, 38]. It is worth mentioning that temperature also
plays an important role during the vitrification, regulating the flow of CPAs
into the cells [39]. Indeed, in this study, vitrification was performed at room
temperature, using ethylene glycol and DMSO as CPAs. Kitazato’s protocol suggests
maintaining blastocysts in VS within 1 minute (45–60 seconds), while the time in
ES generally fluctuated between 5 and 15 minutes, which agrees with several
published articles [5, 6, 10, 11, 40, 41]. Animal studies have reported
contrasting results on this topic. Kader and colleagues [22] evaluated the impact of
equilibration time on the DNA integrity of vitrified-warmed mouse blastocysts.
They recommended an equilibration time of 8 minutes at room temperature to
improve mouse blastocyst DNA integrity [22]. Conversely, Bagis and collaborators [23]
found that vitrification with a 15 minutes equilibration resulted in a higher
hatched blastocyst rate compared to that seen at 5 or 10 minutes. Recently,
Berteli and co-authors [38] analyzed about 1000 vitrified mouse oocytes, aiming
to define the effect of the exposure time to ES on lipid characterizations and
future embryonic development. They found that a longer equilibration time (10
minutes) produced lower oocyte survival and blastocyst rates compared to 7
minutes of exposure. As such, these authors concluded that a longer equilibration
phase pre-vitrification can impair embryo development and cause modification in
oocyte lipid composition, associated with membrane integrity [39]. Divergent
results have also been found in humans, where some reports adopted a fixed
equilibration time of 5 minutes [24, 25], while others increased the
equilibration phase pre-vitrification to 10 [26] or up to 15 minutes [27, 28].
Xiong and colleagues [42] analyzed the topic in 517 frozen-warmed human embryos. They
split the cycles into four groups according to the equilibration time: 5–6 min,
6–7 min, 9–10 min, and 11–12 min, and found no differences in terms of
survival rate between the groups. But implantation and live birth rates were
lower in the 5–6 minutes exposure group compared with the three other groups.
However, the mentioned study was performed on cleavage-stage embryos and our
study is performed on blastocysts, which responded differently to the permeation
of CPAs [42]. Mitsuhata and collaborators [43] reviewed 80 non-expanded and 112
expanded blastocysts and applied two equilibration times pre-vitrification: 8–11
and 12–15 minutes. They found no difference between the two groups in survival,
implantation, and live birth rates, which agrees with our results. However, the
authors reported a significantly improved outcome for the expanded blastocysts in
the 12–15 minutes group compared to the 8–11 minutes group [43]. However, in
the cited study, expanded blastocysts were defined when the blastocoels occupied
greater than half of the embryo volume with a diameter
In an animal model study, different times in ES influence the abortion rate. The possible mechanisms could be correlated with several reasons, such as DNA damage and fragmentation as demonstrated elsewhere [22]. Spindle abnormalities were observed in vitrified blastocyst compared with fresh blastocyst [50], severe changes in temperature, and osmotic stress [17, 35], as well as damage induced by exposure to high concentrations of CPAs [18, 19]. Preliminary studies analyzing this concern have reported that cell exposure to high concentrations of DMSO might induce epigenomic alteration, as well as impair cellular metabolism, cell growth, and apoptosis [19, 20, 21, 51, 52, 53, 54, 55]. In our study, the miscarriage rate was lower in the 6–7 minutes group than in the 9–10 minutes group, however, data displayed no significant difference in the two groups.
Additional studies, especially large-scale epidemiological reports are urgently needed to further understand the implications that cryopreservation and high concentration of CPA might have on the health of children conceived following ART, not only at the time of delivery but also during adult life. Particularly noteworthy is the increase in macrosomal and large for gestational age (LGA) newborns, in addition to a decrease in low birth weight (LBW) and small for gestational age (SGA) newborns [56].
However, no significant differences have been found between group A and group B in terms of neonatal outcomes. The current study carries the limitation of its retrospective design. This is not a randomized controlled trial, it is a retrospective observational cohort study aiming to calculate the effect of different ES time pre-vitrification of artificially collapses blastocyst on the survival and pregnancy rates, as well as neonatal outcomes. In addition, the presence of potential confounding factors due to the heterogeneous nature of the samples investigated may impair the efficacy of our conclusions. However, the comparison of confounders such as female age, maternal BMI, basal FSH, the number of blastocysts transferred, years of infertility, and endometrial thickness were not statistically different between the two groups. Furthermore, it is worth mentioning that the manipulation skills of each embryologist may influence the overall vitrification process, but in this study, vitrification and warming were performed by only two experienced embryologists and their performance were similar each year. Thus, we do believe that variations in technique between operators presumably did not influence the results. In our study, open vitrification system was used to allow direct contact of biological samples with LN2. Gullo et al. [57] showed that there was no statistically significant difference between closed and open vitrification with regards to survival, implantation, clinical pregnancy, and live birth rates.
As far as COVID-19 is concerned, the pandemic has seriously affected the lives of the global population. While the novel coronavirus has broad health implications across the globe, being overlooked in response and policy debates is the impact on women’s reproductive health. In 2021, Owens et al. [58] reported that the COVID-19 pandemic has significantly impacted the reproductive health of women. However, women undergoing ART after the COVID-19 pandemic exhibited no significant difference in the clinical pregnancy rate, miscarriage rate, embryo cryopreservation rate, and other clinical outcomes [59]. Furthermore, patients who were infected by COVID-19 were not included in the study.
To conclude, our findings demonstrate that laser collapse of blastocysts before vitrification and shorter equilibration time of 6–7 minutes leads to similar clinical and neonatal outcomes. However, our results still require further investigations and prospective studies to confirm the benefit of shorter ES time as a routine protocol to improve the efficacy of the vitrification process.
The original data of individual participants underlying this article will be shared on reasonable request to the corresponding author.
LGZ conceived the idea, designed the study, conducted the analysis, and wrote the manuscript. YHL, NL designed the figures and tables. RS designed the figures and tables and revised the manuscript. All authors participated in the discussion of analysis and interpretation of data in this article. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All participants provided written informed consent. Furthermore, the study was approved by the Ethics Committee of Assisted Reproductive Medicine at the Haikou Mary Hospital (Ethics approval number: 2021-10-02).
We thank the clinical staff of our centers for their help in the clinical work which made this study possible.
Project support was provided by the Medical and Health Research Project of Hainan (2101320319A2003).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
