- Academic Editor
Background: This study aims to present a novel technique that
integrates intraoperative neurophysiological monitoring (IONM) into laparoscopy
for continuous monitoring of pelvic nerves at risk during surgery to ensure their
protection. Methods: This is a prospective analysis of 10
consecutive patients receiving surgical treatment for proven diagnosis of
lumbosacral plexus nerve entrapment. Patients with symptoms of chronic pelvic
pain, dyspareunia, dysmenorrhea, and severe, burning sharp pain on the lower
extremity dermatomes were included. Laparoscopic decompression of lumbosacral
plexus nerve entrapment with intraoperative neuromonitoring was performed between
January 2021 and February 2022. Intraoperative neuromonitoring records
(spontaneous electromyography (EMG), free-run EMG recordings, transcranial electrical motor-evoked
potentials (TcMEP) recordings, direct nerve root stimulation recordings, and
compound muscle action potentials (CMAPs) recordings) and preoperative and
postoperative pain symptoms at one month were analyzed. Results:
The median age of the patients was 29 (25–44) years. Neurovascular conflict,
fibrosis, and abnormal piriformis muscle were identified as the three main
etiologies of nerve entrapments. There were no statistically significant
differences in transcranial motor evoked potential responses on the
operated extremity side before and after decompression surgery or in the
amplitude difference changes of TcMEP responses between the operated and
non-operated extremity sides (p
Intraoperative neurophysiological monitoring (IONM) is an electrophysiological method which monitors functional integrity of peripheral or central nervous system during surgery. The IONM procedure is effective in preventing or decreasing the neurological injury near the operative field and may allow for corrective actions to be implemented to prevent permanent deficits, thus improving safety and surgical outcomes. Some examples include IONM of the glossopharyngeal and vagus nerves during surgeries involving the lower brain, intraoperative neuromonitoring during sciatic nerve schwannoma excision, and intraoperative monitoring of the femoral nerves in total hip arthroplasty surgery [1, 2, 3].
Increasing interest of neuropelviology science has significantly increased the awareness of pelvic nerve pathologies. Entrapment neuropathies stem from the compression and irritation of peripheral nerves as they traverse through anatomical passageways. Intrapelvic entrapment of lumbosacral plexus or sciatic nerve can cause chronic pelvic pain, dyspareunia, dysmenorrhea and severe, burning sharp pain on the lower extremity dermatomes. When a nerve becomes trapped, pain symptoms extend along the path of the nerve’s dermatome. Vascular entrapment, fibrotic tissue, aberrant piriformis muscle bundles and endometriosis are among the most common causes of intrapelvic nerve entrapment [4, 5, 6].
Laparoscopic surgery is a minimally invasive surgery that offers several advantages over traditional open surgery. It has the advantages of, less pain, smaller abdomial scars, fewer wound complications, quicker return to activities, and shorter duration of hospital stay. It is difficult to reach the sacral nerve roots, sciatic nerve and pudendal nerve areas in the pelvis surgically, in these cases laparoscopic surgery facilitates the approach to these areas [7].
Since the use of IONM has gained increasing popularity in different surgical disciplines, for the first time in the literature, we described a novel technique by integrating the IONM into laparoscopy to protect pelvic nerves by continuously monitoring when they are at risk during surgery.
This is a prospective analysis of 10 consecutive patients receiving surgical treatment for proven diagnosis of lumbosacral plexus nerve entrapment. All women admitted to our clinic with symptoms of chronic pelvic pain, dyspareunia, dysmenorrhea and severe, burning sharp pain on the lower extremity dermatomes, which had been present for at least one year. All women underwent a laparoscopic decompression of lumbosacral plexus nerve entrapment with intraoperative neuromonitoring system by one surgeon (Ahmet Kale) between December 2021 and February 2022, Health Science University, Kartal Dr. Lutfi Kırdar Education and Research Hospital, Neuropelviology center (Istanbul, Turkiye). Local Ethics Committee approved the study protocol (2022/514/220/8) and informed consent was obtained from all patients. The study was registered at https://clinicaltrials.gov (registration number NCT06009640).
In our study, our approach to decision-making was multi-faceted, encompassing symptoms, neuropelveological evaluation, medical history, diagnostic test, patient preferences, and the current literature. In order to comprehensively characterize preoperative symptoms linked to neurovegetative dysfunctions, we adhered to the guidelines outlined in the International School of Neuropelveology (ISON) manual. Preoperative neuropelveological examination was performed to diagnose lumbosacral plexus nerve entrapment following the criteria of ISON school [8]. After a thorough examination, we evaluated vascular and/or muscle-related nerve compression in the sciatic nerve, sacral nerve roots, and pudendal nerve utilizing a 3 Tesla magnetic resonance imaging (MRI) (3T Prisma, Siemens, Germenger, Germany), chosen for its enhanced visualization of pelvic nerve planes. In each of the ten cases exhibihited clear evidence of neurovascular conflicts in the preoperative MRI scans. Surgical intervention was reserved for patients identified as appropriate candidates following meticulous screening.
Clinical and surgical data, including: demographic characteristics (age, body mass index (BMI), parity), intraoperative neuromonitoring records (spontaneous electromyography (EMG) (free-run EMG recordings, transcranial electrical motor-evoked potentials recordings, direct nerve root stimulation recordings, and compound muscle action potentials (CMAPs) recordings), preoperative and postoperative pain symptoms at one month (dysmenorrhea, dyspareunia, sciatic pain, chronic pelvic pain), operative outcomes (operative time, hemoglobine drop after surgery at 24 hours, intra and postoperative complications) were analyzed for the study purpose.
Intraoperative neurophysiological monitoring records (L5, S1, S2, S3 and S4 nerve roots) were taken just before the operation started and as soon as the operation finished. Electrophysiological recordings were obtained using an Nicolet Endeavor CR IOM Machine (C131205004, Natus Medical Incorporated, Middleton, WI, USA). For the intraoprative neuromonitoring of lumbosacral nervous structures, three modalities were applied on all patients: spontaneous EMG (free-run EMG), TcMEP and direct nerve root stimulation. Compound muscle action potentials were recorded through standard intramuscular needle electrodes, which were placed bilaterally from the tibialis anterior (L5 root), the medial gastrocnemius (S1–2 roots, primary S1), the abductor hallucis (S1–3, primary S2), and the external anal sphincter (S2–4 root) muscles. The recording and filtering parameters were 30- to 1000-Hz band-pass filter; 100-msec analysis time; 10–100 uV/div gain (Fig. 1B–E).
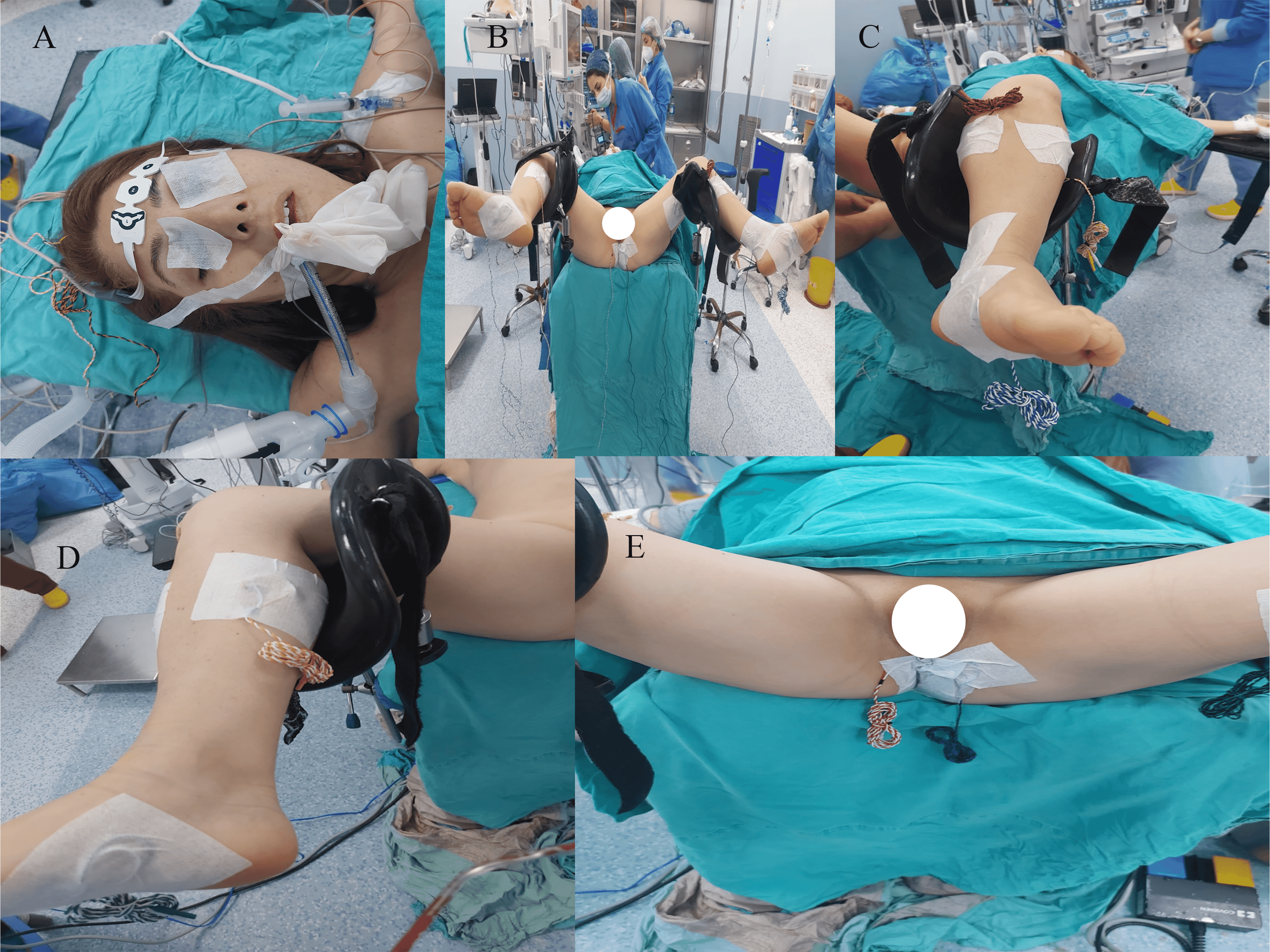 Fig. 1.
Fig. 1.Intraoperative Neurophysiological Monitoring (IONM) Technique. (A) Needle electrodes were inserted subcutaneously at for transcranial electrical stimulations. (B) Needle electrodes were placed bilaterally on the tibialis anterior, the medial gastrocnemius, the abductor hallucis muscles to record compound muscle action potentials (CMAPs). (C) Close-up view of needle electrodes on the left lower extremity. (D) Close-up view of needle electrodes on the right lower extremity. (E) Needle electrodes were placed at the external anal sphincter muscles to record compound muscle action potentials.
For transcranial electrical stimulations, needle electrodes were inserted subcutaneously at C3 and C4 positions according to the international 10-20-EEG system. Anodal stimulation was administered to the motor cortex, then the polarity was switched to stimulate the contralateral motor cortex. Myogenic motor evoked potentials (MEPs) were derived using a constant voltage pulse train (five-seven pulses, 4 ms apart; intensity, 200–400 V; duration of each pulse, 0.5 ms). After anesthesia induction and intubation, baseline TcMEP recordings were obtained before starting the pelvic dissection. Recordings were repeated during the intraoperative period and closure (Fig. 1A and Fig. 2A,B).
 Fig. 2.
Fig. 2.TcMEP recordings. (A) Baseline. (B) The end of laparoscopic decompression surgery. Compound muscle action potentials (myogenic motor evoked potentials) recorded from the bilateral lower extremity and unilateral EAS muscles following transcranial electrical stimulation in a patient with a diagnosis of lumbosacral plexus nerve entrapment. TcMEP, transcranial electrical motor-evoked potentials; AH, abductor hallusis; EAS, external anal sphincter; MG, medial gastrocnemius; TA, tibialis anterior.
Free-run EMG was used to detect spontaneous motor unit discharges produced from motor nerves due to damage or manipulation of sacral neural tissues. Moreover, we mapped afferent myotomal distribution covering each nerve root from L5 to S4 at the surgical site by applying the direct nerve root stimulation with a disposable bipolar direct nerve stimulator probe (Figs. 3,4). CMAPs elicited with stimulus intensities less than 10 V (generally 3–5 V) were interpreted as being near a nerve root, probably with intervening tissue. The constant-voltage duration was 0.2 ms and the rate was 3 Hz. Stimulation was started at 0.5 V and then increased gradually (0.1–0.3 V step) depending upon the CMAPs obtained.
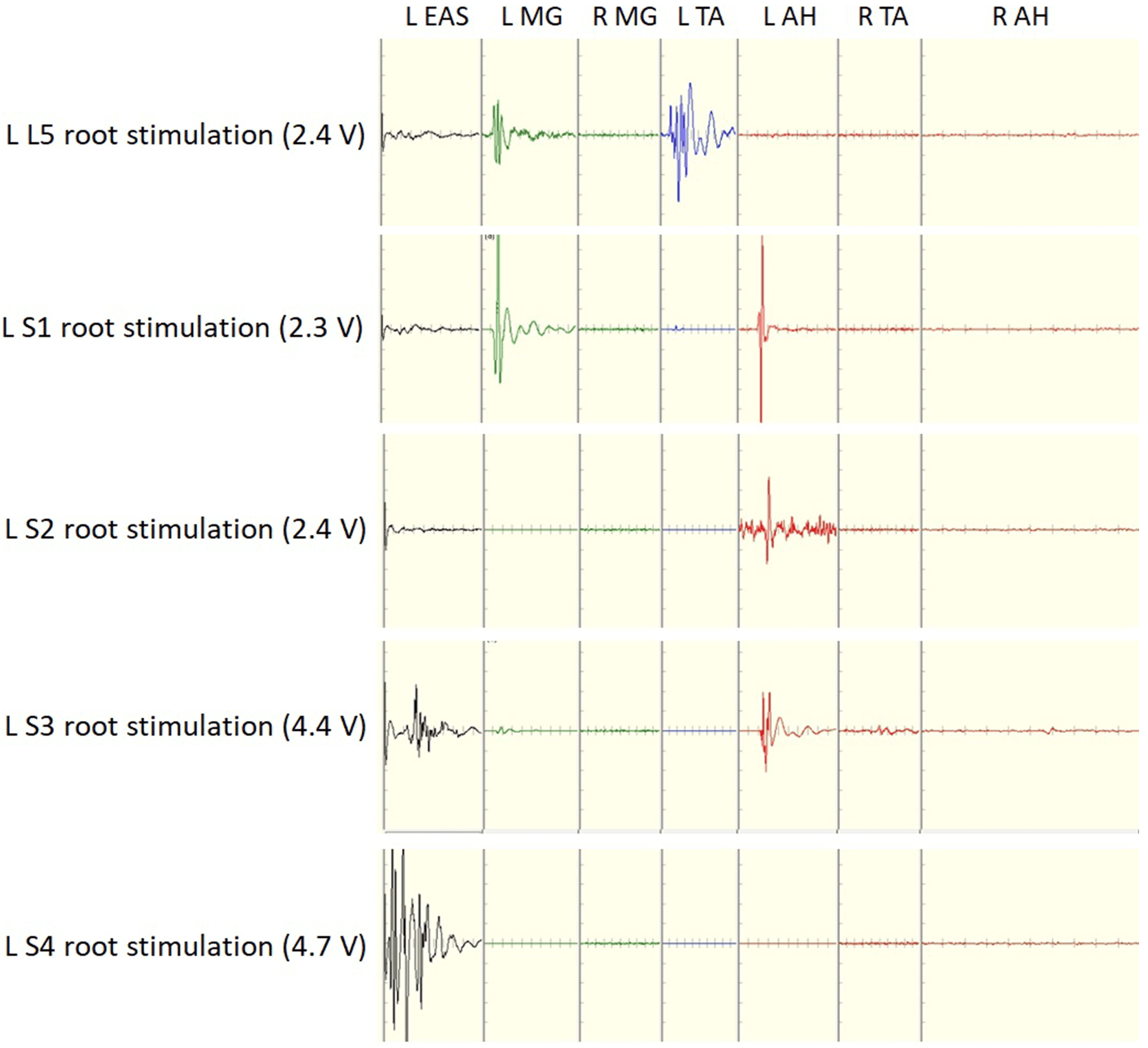 Fig. 3.
Fig. 3.Recordings of compound muscle action potential obtained by direct electrical stimulation of lumbosacral roots at stimulus intensities 2.4 and 4.7 V.
 Fig. 4.
Fig. 4.Laparoscopic view of direct nerve stimulations. Compound muscle action potentials recording with using disposable bipolar direct nerve stimulator probe from L5 to S1 nerve roots during laparoscopic decompression surgery. (A) L5 nerve root stimulation with direct nerve stimulator probe. (B) S1 nerve root stimulation with direct nerve stimulator probe. (C) S2 nerve root stimulation with direct nerve stimulator probe. (D) S3 nerve root stimulation with direct nerve stimulator probe. (E) S4 nerve root stimulation with direct nerve stimulator probe.
We have previously described a surgical technique in our published articles where we performed laparoscopic sacral plexus exploration for nerve decompression [6].
Summarizing the surgical approaches: Initially, the patient underwent routine preparations before the commencement of laparoscopic procedures Preoperative basal neurophysiological monitoring records (TcMEP recordings) were taken just before the operation. Following laparoscopic exploration, the initial action involved using bipolar cautery and mono polar scissors to establish a broad peritoneal incision between the external iliac vessels and the obliterated umblical ligament on the front side of the pelvic wall. The dissection was extended further between the medial edge of the iliopsoas muscle on side of the pelvis and the external iliac vessels, in accordance with the earlier description. Continuing with the procedure, the following step involved pinpointing the presence of the obturator nerve within the confines of the obturator fossa. At the depth of the iliolumbar fossa, access was gained to several vessels with multiple branches that were in proximity to the lumbosacral trunk. Utilizing a LigaSure vessel-sealing bipolar electrosurgical device (Blunt Tip; Covidien, Dublin, Ireland), the vessels with multiple branches were cauterized and cut. For the third step, the dissection was extended downward, revealing enlarged vessels with multiple branches. These vessels were meticulously isolated from the sciatic nerve and sacral nerve roots, and dissected using laparoscopic grasper (Karl Storz, Tuttlingen, Germany) along with cold scissors. Intraoperative neurophysiological monitoring was performed to prevent damage to the sacral nerve roots simultaneously while intervening the vessels on the sciatic nerve and sacral nerve roots. Hem-o-Lok clips (Weck Closure System; Teleflex, Wayne, PA, USA) were used to secure the aberrant artery and veins that traversed over the sciatic nerve and sacral nerve roots. The LigaSure vessel-sealing device was utilizes to detach the vessels at both their proximal and distal points. Once all anomalous vessels situated above the lumbosacral trunk were removed, clear visualization of the sciatic nerve and sacral nerve roots was visualized. Upon concluding the procedure, it was observed that the sciatic nerve and sacral nerve roots had been fully freed. Subsequent to the surgery’s completion, neurophysiological monitoring records (L5, S1, S2, S3 and S4 nerve roots) were promptly collected (Figs. 5,6).
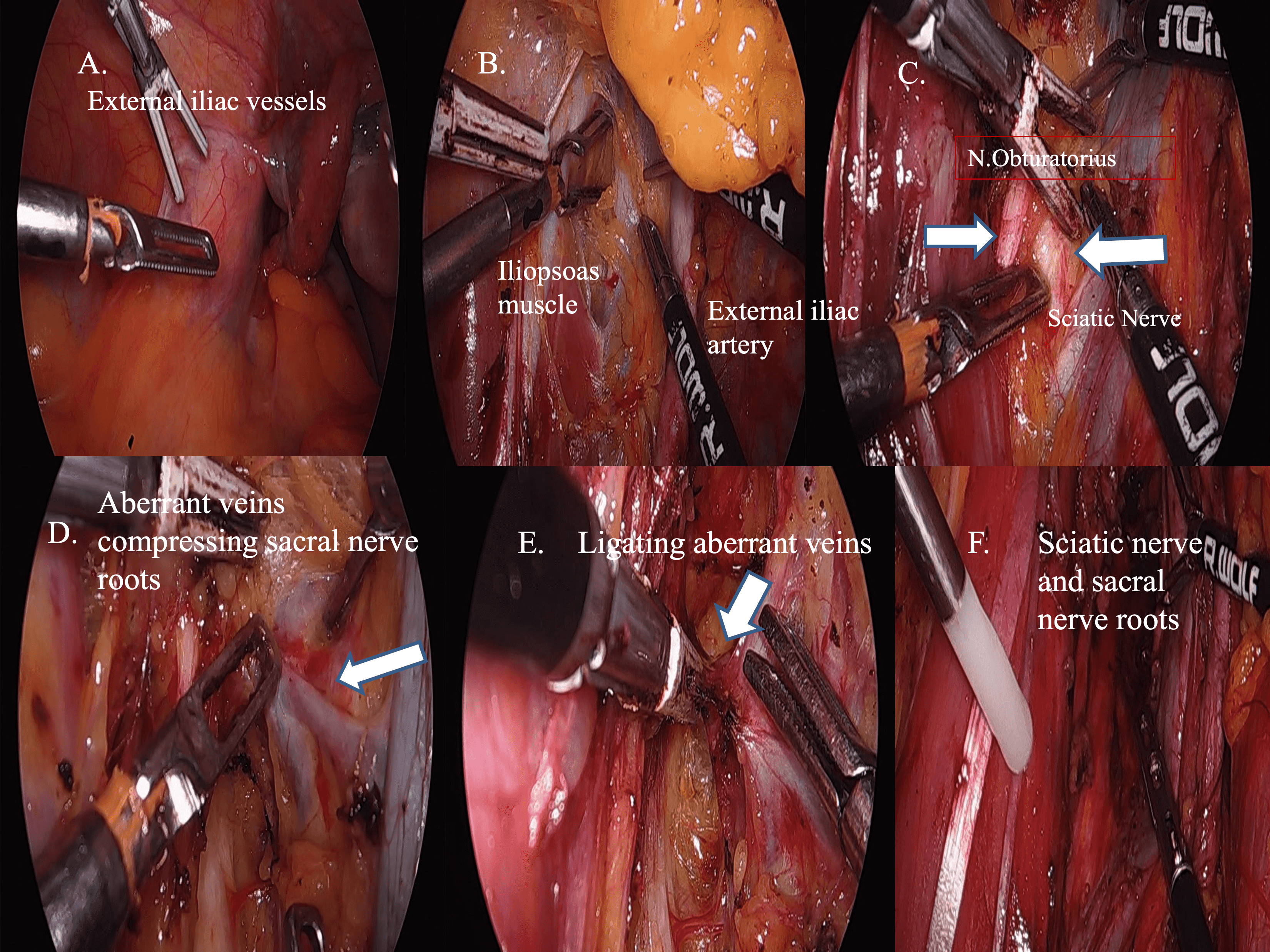 Fig. 5.
Fig. 5.Postoperative neurophysiological monitoring records (L5, S1, S2, S3 and S4 nerve roots). (A) External iliac vessels and pelvic side wall. (B) Creating a broad peritoneal incision spanning from the external iliac vessels to the iliopsoas muscle. (C) The dissection was deepened between the external iliac vessels and iliopsoas muscle to show the obturator and sciatic nerve. (D) Multiple-aberrant branching vessels that were compressing lumbosacral trunk and sacral nerve roots. (E) The ligature vessel-sealing device was employed to delicately isolate and disconnect the multiple-branching enlarged vessels from the sciatic nerve and sacral nerve roots through careful skeletonization. (F) Upon completion of the procedure, complete liberation of the sciatic nerves and sacral nerve roots was achieved.
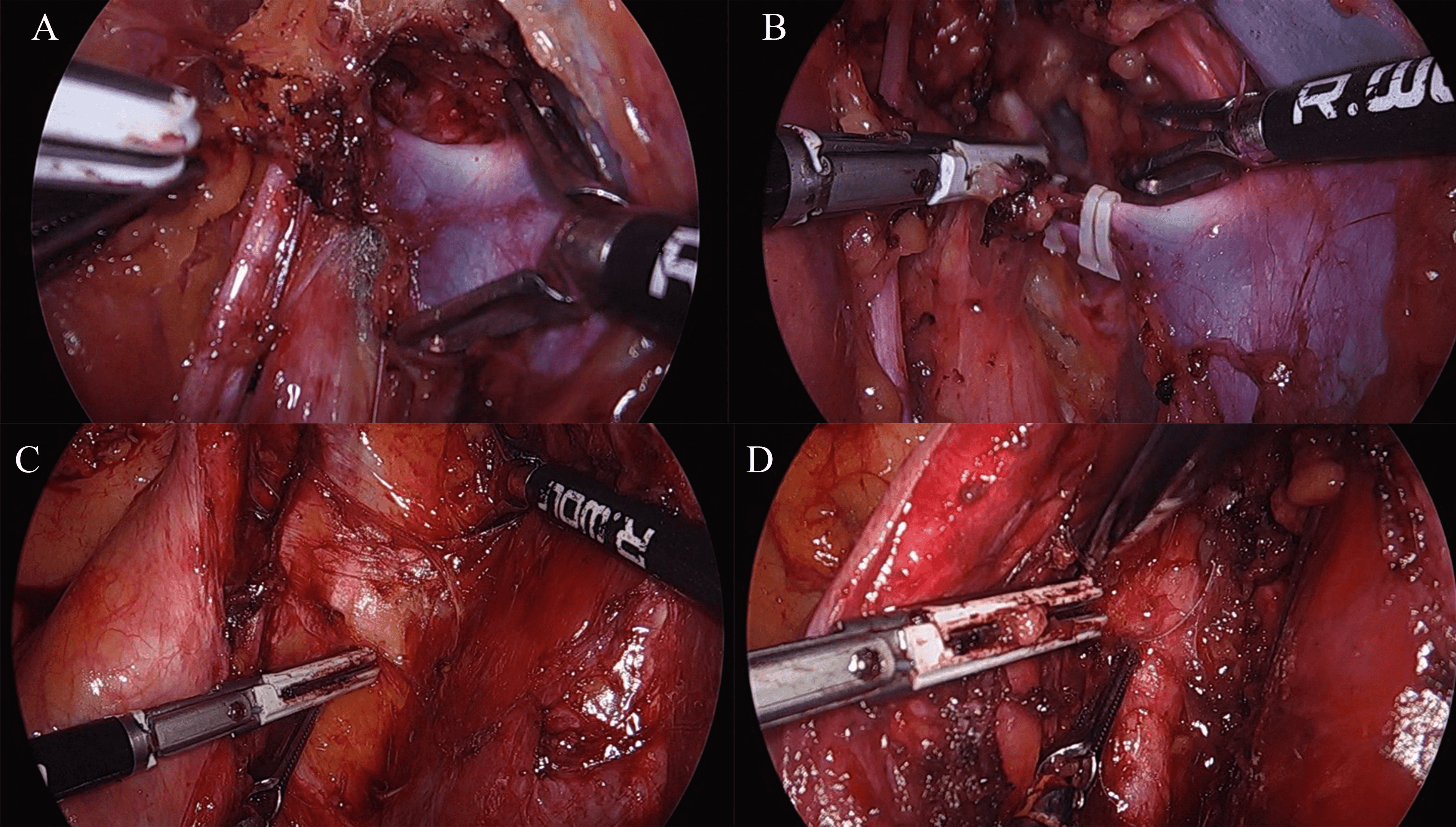 Fig. 6.
Fig. 6.Shows laparoscopic surgical intervention of etiological factors causing nerve compression. (A) Abnormal vein which was compressing over sacral nerve roots. (B) Abnormal vein was clipped using Hem-o-Lok clips, cut and decompressed using the LigaSure vessel-sealing device. (C) Fibrous tissue was compressing over sacral nerve roots. (D) Fibrous tissue was cut and decompressed using the LigaSure vessel-sealing device.
Pain symptoms (dysmenorrhea, dyspareunia, dyschezia, sciatic pain, chronic
pelvic pain) were evaluated preoperatively, and re-evaluated at the postoperative
first month. Visual analogue scale (VAS) were used in pain score assessment. The
Visual Analogue Scale used to measure pain intensity, with 0 denoting no pain and
10 indicating the most severe pain imaginable. The clinical significance of pain
reduction was defined as a numeric rating scale decrease of
Postoperative complications and any motor deficit within the myotomes of the lumbosacral plexus were assessed both immediately after surgery and again at the one-month mark.
The main objective of this study was to prevent or minimize the risks of sciatic and sacral nerve roots injury in laparoscopic decompression surgery with using the IONM. The secondary outcome of the study involved assessing clinically significant pain reduction one month post-surgery.
Analysis of the data was carried out using the SPSS Version 25.0 (IBM Corporation, Chicago, IL, USA) package program. Frequency and precentage values for qualitative varaibles, median, minumum and maximum values for quantative varaibles are presented. Wilcoxon test was used to compare quantative measurements with two replicates, and the Friedman test was used to compare quantative measurements with more than two replicates, type 1 error rate was taken as 0.05 in the study.
During the study duration, a total of 10 female patients underwent laparoscopic
nerve root decompression surgery accompanied by intraoperative neurophysiological
monitoring. The median age of the patients was 29 (25–44) years in this study
(Table 1). The median BMI, and parity of the patients were as 26.76 (23.34–30.8) kg/m
| Med (min–max) | Complications | Motor deficit | |
| Age (years) | 29 (25–44) | - | - |
| BMI (kg/m |
26.76 (23.34–30.8) | - | - |
| Parity | 2 (0–3) | - | - |
| Operating time (min) | 57.5 (45–70) | - | - |
| Hemoglobine drop after surgery at 24 h (g/dL) | 0.85 (0–2.20) | - | - |
| Intraoperative complications | - | None | - |
| Postoperative complications | - | None | - |
| Motor deficit immediately after surgery | - | - | None |
| Motor deficit at the first month after surgery | - | - | None |
BMI, body mass index.
In the course of laparoscopy, a comprehensive evaluation was undertaken for each patient to detect potential comorbidities, such as peritoneal endometriosis. Our attention extended beyond nerve-related pathologies to encompass concurrent health concerns that could impact both the patient’s general health and surgical results. It’s noteworthy that no instances of comorbidities were identified within the subset of patients included in our study.
No intraoperative and postoperative complications developed. Bilateral lower extremity motor functions were normal immediately after the operation and in the first postoperatively (Table 1).
Table 2 provides an overview of distribution of nerve entrapments locations, etiological factors, and pain localization on dermatomes. The prevailing entrapment sites were observed to be the left proximal S2/S3/S4 nerve roots in 30% of cases and the right proximal S2/S3 nerve roots and sciatic nerve in a equal percentage of 30% cases. This was followed by occurrences at the left distal S3/S4 distal nerve roots (10%), right proximal S1/S2 nerve roots (10%), right proximal S2/S3 nerve roots (10%) and left proximal S2/S3 nerve roots (10%). Among these 10 patients, surgical exploration revealed three distinct causes for nerve entrapments: neurovascular conflict, fibrosis, and an anomalous piriformis muscle. Neurovascular conflict and fibrosis were the most common etiologies of entrapment in this group of patients (50% and 40%, respectively), followed by abnormal piriformis muscle entrapment (10%). Specifically, the only case that exhibiting an abnormal piriformis muscle had muscular hypertrophy.
| Distrubition of nerve entrapment locations | Etiology | Pain dermatomes | Number of patients |
| Left proximal S2/S3/S4 nerve roots | Neurovascular conflict | S2, S3, S4 | 3 patients |
| Right proximal S2/S3 nerve roots and sciatic nerve entrapment | Fibrosis | S2, S3 | 3 patients |
| Left distal S3/S4 distal nerve roots | Neurovascular conflict | S3, S4 | 1 patient |
| Right proximal S1/S2 nerve roots | Neurovascular conflict | S1, S2 | 1 patient |
| Right proximal S2/S3 nerve roots | Abnormal piriformis muscle entrapment | S2, S3 | 1 patient |
| Left proximal S2/S3 nerve roots | Fibrosis | S2, S3 | 1 patient |
Spontaneous EMG activity was present in 6 patients (60%). In 4 cases (40%) no EMG activity was recorded in any EAS or lower limb muscle throughout the surgical procedure. In each for 10 patients, reproducible CMAPs evoked by direct stimulation of lumbosacral nerve roots were recorded from the EAS and lower limb muscles on the symptomatic side. Recording of CMAPs in one muscle or simultaneously in two or three of the monitored muscles after stimulation of the nerve roots from L5 to S2 was successful in all 10 patients. In 2 (20%) of these patients no triggered CMAPs were obtained with S3 root stimulations in either the EAS or lower limb muscles. There were 5 (50%) patients in whom no CMAPs were elicited with S4 root stimulation even at 10 V. Postoperative sensory or motor deficits were not noted in any of these patients. The identification of S3 and S4 nerve roots on visual inspection had been insufficient probably due to anatomical variations. The findings of direct stimulation to each nerve root are summarized in Table 3.
| Root | Stimulus intensity (Median, range) | Tibialis anterior (n, %) | Medial gastrocnemius (n, %) | Abductor hallucis (n, %) | External anal sphincter (n, %) |
| L5 root (n = 10) | 3.50 V (1.9–10 V) | 10 (100) | 4 (40) | 1 (10) | None |
| S1 root (n = 10) | 3.80 V (1.9–10 V) | None | 8 (80) | 5 (50) | None |
| S2 root (n = 10) | 4.65 V (1.9–10 V) | None | 4 (40) | 9 (90) | 2 (20) |
| S3 root (n = 8) | 4.65 V (1.9–10 V) | None | None | 1 (12.5) | 8 (100) |
| S4 root (n = 5) | 4.70 V (2.70–10 V) | None | None | 1 (20) | 5 (100) |
There was no statistical difference with regard to TcMEP responses on the non-operated extremity side before and after
surgery in all patients (p
| Preoperative | Postoperative | Z | p | |
| TA_amplitude (V) | 37.75 (27.8–42.6) | 42.35 (35.6–47.8) | –1.886 | 0.059 |
| MG_amplitude (V) | 37.15 (33.6–47.4) | 37.55 (31.6–42.5) | –0.968 | 0.333 |
| AH_amplitude (V) | 44.4 (28.6–57.4) | 40.45 (28.5–52.6) | –1.120 | 0.263 |
| EAS_amplitude (V) | 35.9 (22.5–55) | 35.85 (18.9–45.6) | –0.420 | 0.674 |
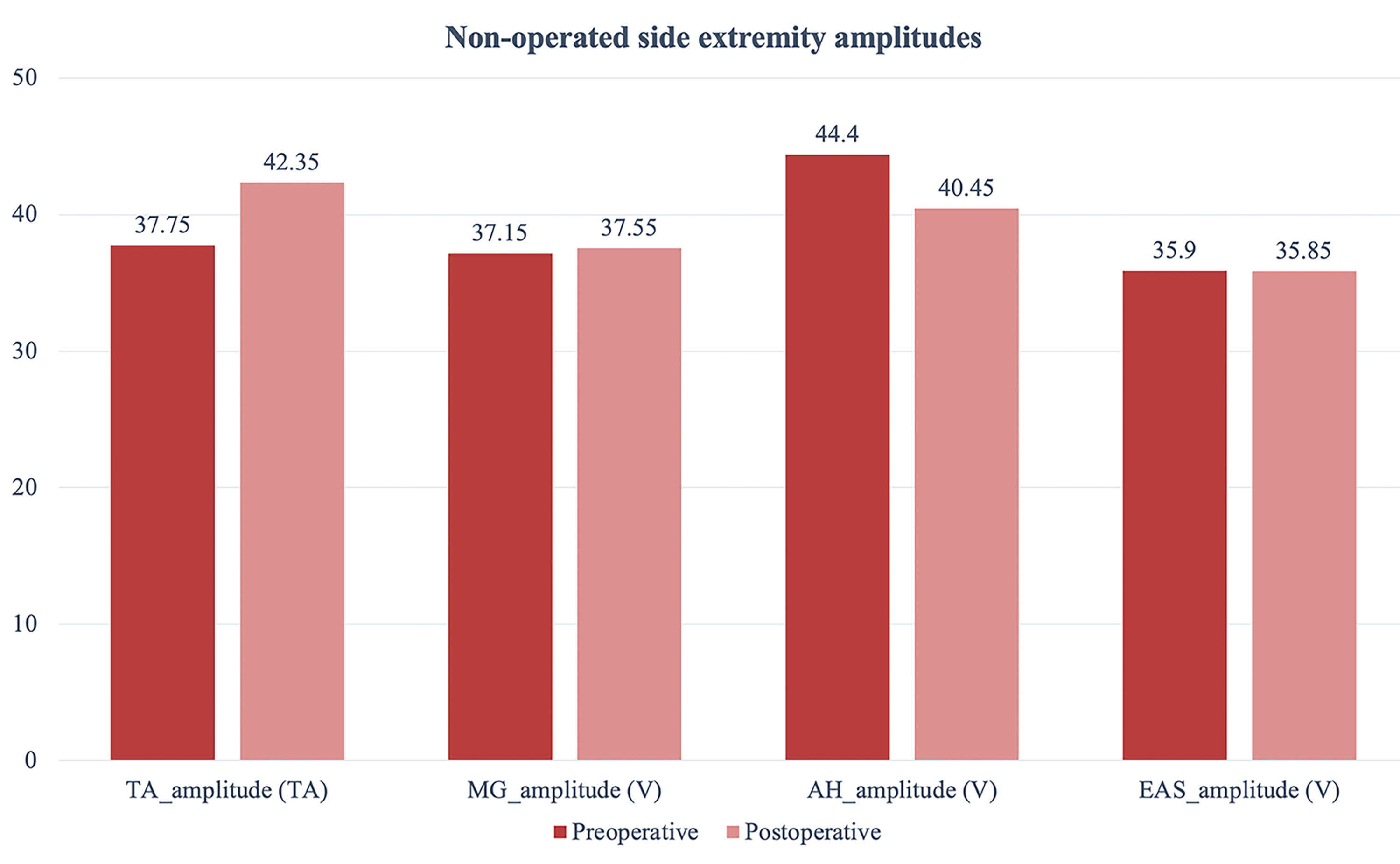 Fig. 7.
Fig. 7.Shows pre-and postoperative non-operated side extremity amplitude values.
There was no statistical difference with regard to TcMEP responses on the
operated extremity side before and after surgery in all patients (p
| Preoperative | Postoperative | Z | p | |
| TA_amplitude (V) | 34.25 (28.9–44.3) | 39.55 (28.6–45.5) | –1.478 | 0.139 |
| MG_amplitude (V) | 34.05 (27.5–41.6) | 38.5 (28.5–44.6) | –1.481 | 0.139 |
| AH_amplitude (V) | 39.4 (22.4–49.8) | 38.1 (18.3–53.1) | –0.663 | 0.507 |
| EAS_amplitude (V) | 34.65 (20.5–52.4) | 43.7 (23.1–48.9) | –1.120 | 0.263 |
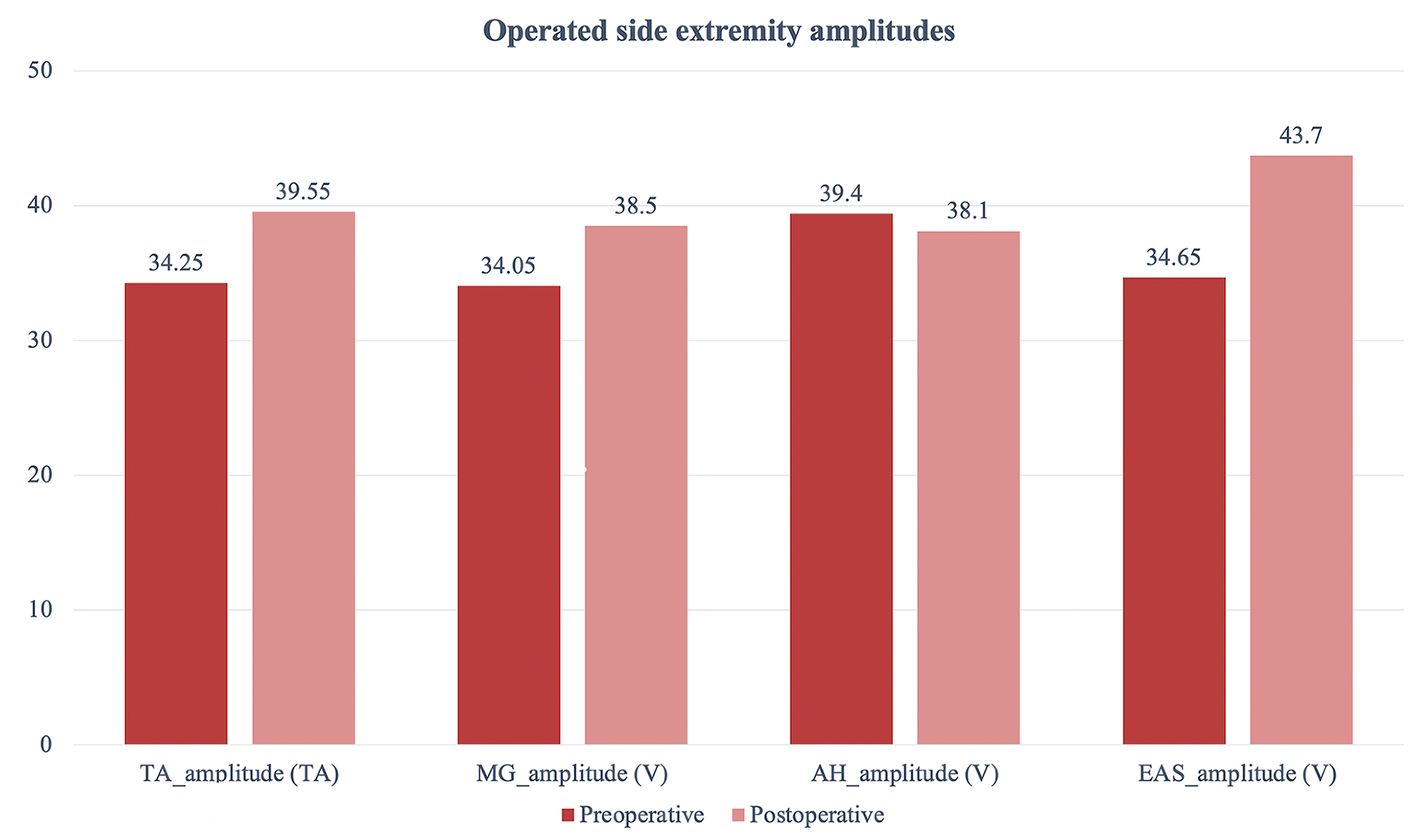 Fig. 8.
Fig. 8.Shows pre-and postoperative operated side extremity amplitude values.
There was no statistical difference with regard to amplitude difference changes
of TcMEP responses on the operated and non-operated extremity side before and
after surgery in all patients (p
| Non-operated extremity side | Operated extremity side | U | p | |
| Difference TA_amplitude (V) | 12.02 (–14.32–42.19) | 4.18 (–23.7–51.56) | 44 | 0.684 |
| Difference MG_amplitude (V) | –4.03 (–23.39–15.77) | 7.35 (–18.18–39.86) | 24 | 0.052 |
| Difference AH_amplitude (V) | –12.97 (–26.17–18.66) | 10.22 (–51.33–62.54) | 27 | 0.274 |
| Difference EAS_amplitude (V) | –5.64 (–46.73–39.93) | 22.27 (–30.27–82.46) | 20 | 0.234 |
Significant reduction in dysparenuia VAS scores was observed at the one month follow-up after the surgery (p-value = 0.027). Sciatic pain VAS scores did not exhibit a significant decrease within the first month following surgery (p-value = 0.505). Although there was a significant decrease in chronic pelvic pain and dysmenorrhea VAS scores at the first month postoperatively, it did not reach statistical significance (p-value = 0.106 and p-value = 0.207 respectively) (Table 7).
| Symptoms | Preoperative Median VAS (range) | Postoperative VAS scores | p-value |
| At the first month (range) | |||
| Sciatic pain | 6.5 (0–10) | 7 (0–9) | 0.505 |
| Chronic pelvic pain | 8 (0–10) | 4.5 (0–9) | 0.106 |
| Dysmenorrhea | 8 (0–10) | 0 (0–10) | 0.207 |
| Dyspareunia | 7.5 (0–10) | 0 (0–9) | 0.027 |
VAS, visual analogue scale.
Minimal invasive pelvic neuromonitoring has been a subject of research providing in combination with endoscopic magnification effect into neuroanatomical distribution of nervous tissue in the pelvic area. The need for actual nerve monitoring has only recently been proposed as a procedure during pelvic surgery to preserve postoperative urinary, sexual and digestive tract dysfunction and therefore simultaneous electromyography devices have applied to provide identification and verification of functional nerve integrity [10].
Laparoscopy enables excellent visibility of pelvic neural structures that are inaccessible for direct viewing. The resolution and magnification effects provide the pelvic surgeon with a safe surgery. Although the effect of laparoscopy in pelvic surgery is significant, seeing the pelvic nerves does not always mean protecting them. It must also be said that nerve identification is not nerve monitoring and visualization of a nerve does not guarantee its postoperative function [7, 10, 11].
Possover et al. [11], introduced the LANN technique, aimed at preserving the pelvic autonomic nervous system in surgeries involving cervical cancer or deeply infiltrating endometriosis. This approach involves the utilization of a laparoscopic bipolar forceps, a conventional stimulator, and intraoperative urodynamic testing.
In our novel technique, we integrated intraoperative neuromonitoring system into laparoscopic lumbosacral plexus nerve decompression surgery and simultaneusly recorded nerve roots from lumbar 5 to sacral 4 during the operation. Diseases that cause pelvic nerve compression such as abnormal vascular conflict, aberrant piriformis muscle, and endometriosis have settled in hard-to-reach deep areas of the pelvis. Laparoscopy facilitates easier access to these regions, offering improved magnification and visualization capabilities. Sacral and sciatic nerves might be damaged when performing decompression surgery in these deep pelvic areas.
Dilated or abnormal branches of iliac vessels could potentially lead to chronic pelvic pain. In their study, Possover et al. [12] conducted laparoscopic exploration of the sacral plexus to relieve nerve compression. Their findings included 37 cases of isolated sacral plexus vascular entrapment and 1 case of piriformis syndrome. In our published studies, we performed laparoscopic intrapelvic nerve decompression utilizing the anterior approach. This technique addressed issues arising from aberrant vessels and a unique piriformis muscle bundle configuration [6]. In this current study, entrapments was primarily attributed to neurovascular conflict and fibrosis. The two most frequent entrapment locations were the left proximal S2/S3/S4 nerve roots in 30% of cases, and the right proximal S2/S3 nerve roots and sciatic nerve in another 30% of cases.
TcMEPs, which are muscle action potentials elicited by transcranial brain stimulation, are used to monitor descending motor pathway during surgery. By comparing potentials before and after correction, surgeons may prevent postoperative motor deficit [13]. Currently, many different surgical procedures use TcMEP monitoring, including supratentorial craniotomy, spinal cord, and intracerebral hemorrhage (ICH) [14].
In our study, TcMEP responses on the operated extremity side before and after
decompression surgery and amplitude difference changes of TcMEP responses on the
operated and non-operated extremity side before and after decompression surgery
were not statistically different (p
Compound muscle action potential is the simultaneous activation of a group of motor neurons within a nerve bundle by electrical stimulation. The measured response of compound muscle action potential correlates to how many axons are being stimulated. CMAPs are recorded clinically to assess muscle function in cases of suspected nerve entrapment, demyelinating neuropathy, and neuromuscular junction disease [15].
Vetrano et al. [16] reported intraaoperative compound muscle action potentials amplitude changes of 24 patients after neurolysis of peripheral nerves in upper limbs of neuropathies and found a statistically significant difference before and after decompression surgery.
Moreover, we recorded compound muscle action potentials at the surgical site by applying the direct electrical stimulation with bipolar direct nerve stimulator probe at stimulus intensities 2.4 and 4.7 V. The purpose of measuring the compound action potential is to map the lumbosacral nerve roots from the L5 nerve root to S4 during laparoscopic decompression surgery (Table 3, Figs. 1,2,3,4).
Laparoscopic nerve decompression surgery provides 65% reduction in somatic pelvic pain. From etiological point of view, laparoscopic pelvic nerve decompression surgery can have a significant effect on VAS scores in 83% of patients with Alcock’s canal syndrome, 62% of patients with endopelvic lesions and 78% of patients with endometriosis [17].
In our series, dyspareunia VAS scores decreased dramatically at the first month after decompression surgery (Table 7). It’s important to highlight that our study reveals a non-significant reduction in both sciatica and chronic pelvic pain. Notably, our study primarily concentrates on presenting the early results following surgical intervention. While statistical significance may not be evident in the initial postoperative phase, it’s worth emphasizing that our study captures the initial outcomes and maintains a longitudinal follow-up approach. Moreover, we are in the processs of planning a study of long-term results from a more extensive patient series, a step that we anticipate will offer deeper insights not the evolving trends and patterns of pain reduction.
This current study’s scope is constrained by a relatively modest sample size. However, the application of laparoscopic surgical treatment for other nerve entrapment conditions, accompanied by IONM holds potential for a broader demographic.
In conclusion, this new technique of the laparoscopic approach to the pelvic somatic nerves opens a new application of intraoperative pelvic nerve monitoring and electroneurostimulation for laparoscopic surgeons. Integration of intraoperative neuromonitoring into laparoscopic pelvic nerve decompression surgery has the potential to enhance the tracking motor function and possibly mitigate the risk of nerve damage during pelvic nerve decompression surgery. Additionally, future investigations focusing on the effects of laparoscopic pelvic nerve decompression surgery with the integration of IONM might further enhance our understanding of the outcomes derived from this technique.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
ECG: Design of the work, drafting the work, the acquisition, interpretation of data for the work. AK: Design of the work, revising manuscript critically for important intellectual content. MM: Design of the work, interpretation of data for the work, providing substantial contributions to the drafting the manuscript and also revising it critically for intellectual content. VY: Design of the work, interpretation of data for the work, providing substantial contributions to the drafting the manuscript and also revising it critically for intellectual content. UEO: The acquisition, interpretation of data for the work, providing substantial contributions to the drafting the manuscript and also revising it critically for intellectual content. TU: Revising manuscript critically for important intellectual content, providing substantial contributions to the design of the work, interpretation of the data for the study. EK: Design of the work, drafting the work, the acquisition, interpretation of data for the work. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Kartal Dr. Lutfi Kirdar City Hospital’s Ethics Committee approved the study protocol (2022/514/220/8) and informed consent was obtained from all patients.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
