- Academic Editor
Background: To explore the predictive value of vascular endothelial
growth factor (VEGF)-C and D combined with ultrasonic pathological features for
nonsentinel lymph node (NSLN) metastasis in positive sentinel lymph nodes (SLNs)
early-stage breast cancer. Methods: To review the clinical data of 170
SLN-positive early breast cancer patients. We examined VEGF-C and D positive
expression in cancerous and paraneoplastic tissues and counted ultrasound and
pathological features. Results: The rate of VEGF-C and D positivity in
cancer tissues was higher than that in paracancerous tissues (p
As the sentinel lymph node (SLN) is the primary site of metastasis from the primary cancer lesion, assessing its metastatic status helps in the clinical selection of the optimal treatment strategy to maximize the survival benefit for patients [1]. Lymph node metastases play a key role in patient treatment failure and shortened survival. A phase 3 clinical trial noted that SLN-negative patients who underwent SLN resection alone without axillary lymph node dissection (ALND) had similar overall survival and disease-free survival to those who underwent ALND [2]. Subsequently, Donker et al. [3] reached similar conclusions. From this, it can be assumed that not all SLN-positive individuals will benefit from ALND. Currently, there is still some controversy regarding the management of SLN in early-stage breast cancer patients with negative axillary lymph nodes, but pathology suggests the presence of one to two SLN-positive lesions, and patients may not receive ALND for those with postoperative adjuvant radiotherapy or other combination treatment options [4, 5]. Therefore, finding reliable protocols to screen for nonsentinel lymph node (NSLN) metastases at high risk from SLN-positive patients effectively prevents patients from receiving unnecessary ALND.
Vascular endothelial growth factor (VEGF) promotes the production of blood vessels and lymphatic vessels. It also has various functions, such as promoting epithelial cell division and regulating vascular permeability [6]. VEGF-C and -D both belong to the VEGF family, which is widely expressed in early embryos and various organs and tissues. In recent years, studies related to malignant tumours such as oesophageal cancer [7] and squamous lung cancer [8] have pointed to the association of VEGF-C and D with lymph node metastasis. However, their roles in lymph node generation and metastasis of breast cancer are not well defined. This study aimed to investigate the predictive value of VEGF-C and D combined with ultrasound and pathological features for NSLN metastasis to guide the choice of clinical ALND treatment.
We reviewed the clinical data of 170 SLN-positive early breast cancer patients
who were sourced from confirmed patients admitted to Nanchang Third Hospital from
February 2014 to January 2018. All were female, aged 26–67 years, with a mean
age of 48.75
 Fig. 1.
Fig. 1.Flowchart of the study. SLN, sentinel lymph node; COPD, chronic obstructive pulmonary disease; VEGF, vascular endothelial growth factor; NSLN,nonsentinel lymph node; AUC, area under the receiver operating characteristic curve.
(1) Pathological examination confirmed the diagnosis of invasive breast cancer;
(2) Female, aged
(1) Distant metastasis of breast cancer lesions; (2) other malignant tumours or history of previous tumour treatment; (3) history of previous chest surgery; (4) combined with mastitis or other benign breast lesions; (5) severe liver and kidney dysfunction, haematological system, autoimmune system diseases; and (6) chronic obstructive pulmonary disease, pulmonary fibrosis and other diseases that may affect the abnormal expression of VEGF.
GE Voluson E9 (General Electric, Fairfield, CT, USA), Super Sonic Imagine Aixplorer (SuperSonic Imagine, aix en Provence, France) diagnostic ultrasound instrument with a probe frequency of 4 to 15 MHz was used for the examination. The patient’s hands were both above the head, and the breast and axillary area were scanned longitudinally, transversely and radially. Senior sonographers analysed nodal features based on Breast Imaging (BI) Reporting and Data System (RADS) [9], including size (maximum tumour diameter), margins (burr sign, smooth, faint, etc.), presence or absence of calcification and type of calcification (intranodal, extranodal, intraductal), and location (external superior, external inferior, internal superior, internal inferior, areolar area), using the Adler semiquantitative method for blood flow grading (0, I, II, III).
After intraoperative sampling of cancerous and paracancerous tissue for paraffin
sectioning, VEGF-C and D were detected by the immunohistochemistry
streptavidin-perosidase (S-P) method, and the required antibodies were purchased
from Abcam (item number: ab106512, ab137368, Cambridge, UK). The sections were
operated according to the product instructions, and known positive sections and
phosphate buffered saline (PBS) were used as positive and negative controls instead of primary antibodies,
respectively. The cell pulp was brown, and tan staining was used as positive
cells. Cell pulp staining was made according to the 400
Patients were generally anaesthetized and detected by the metabotropic dye tracer method, with 2 mL of metabotropic dye injected into the edge of the areola above the outer breast and fully massaged for approximately 10 minutes. A 3–4 cm axillary incision was made, and all blue-stained lymph nodes were removed. Axillary lymph node dissection was conducted for SLN-positive patients by rapid frozen section and postoperative pathology, including regional lymph nodes I and II [10]. The presence or absence of metastases in NSLN metastasis was clarified on the basis of postoperative pathological findings.
Patient records were collected, and patients’ age (
SPSS 26.0 (Version 26.0, International Business Machines Corporation, Armonk,
NY, USA) was used to analyse the data, and the count data were expressed as
a number or %, the
The positive rates of VEGF-C and D in breast cancer tissues were higher than
those in adjacent tissues (p
| Group | n | VEGF-C | VEGF-D |
| Cancer tissues | 170 | 133 (78.24%) | 105 (61.76%) |
| Adjacent tissues | 170 | 62 (36.47%) | 32 (18.82%) |
| 59.028 | 65.149 | ||
| p |
VEGF, vascular endothelial growth factor.
The rates of VEGF-C and D positivity in cancer tissues of SLN-positive patients
with vascular infiltration, number of SLN positives
| Clinicopathological features | n | VEGF-C positive (n = 133) | p | VEGF-D positive (n = 105) | p | |||
| Age (years) | 1.582 | 0.208 | 0.205 | 0.651 | ||||
| 152 | 121 (79.61%) | 93 (61.18%) | ||||||
| 18 | 12 (66.67%) | 12 (66.67%) | ||||||
| Clinical Staging | 0.250 | 0.617 | 1.229 | 0.268 | ||||
| T1 | 72 | 55 (76.39%) | 41 (56.94%) | |||||
| T2 | 98 | 78 (79.59%) | 64 (65.31%) | |||||
| Pathology Type | 0.014 | 0.907 | 0.037 | 0.846 | ||||
| IDC | 148 | 116 (78.38%) | 91 (61.49%) | |||||
| Other | 22 | 17 (77.27%) | 14 (63.64%) | |||||
| Histology grade | 0.873 | 0.646 | 0.119 | 0.942 | ||||
| 1 | 3 | 3 (100.00%) | 2 (66.67%) | |||||
| 2 | 102 | 79 (77.45%) | 62 (60.78%) | |||||
| 3 | 65 | 51 (21.54%) | 41 (63.08%) | |||||
| Vascular infiltration | 10.485 | 0.001 | 38.225 | |||||
| No | 114 | 81 (71.05%) | 52 (45.61%) | |||||
| Yes | 56 | 52 (92.86%) | 53 (94.64%) | |||||
| SLN number | 0.631 | 0.427 | 0.239 | 0.625 | ||||
| 36 | 27 (72.97%) | 24 (64.86%) | ||||||
| 134 | 106 (79.10%) | 81 (60.45%) | ||||||
| SLN positive number | 10.134 | 0.001 | 22.551 | |||||
| 140 | 103 (73.57%) | 75 (53.57%) | ||||||
| 30 | 30 (100.00%) | 30 (100.00%) | ||||||
| SLN positive percentage | 5.201 | 0.023 | 23.298 | |||||
| 111 | 81 (72.97%) | 54 (48.65%) | ||||||
| 59 | 52 (88.14%) | 51 (86.44%) | ||||||
| Molecular type | 1.558 | 0.816 | 1.437 | 0.838 | ||||
| Luminal A | 30 | 22 (73.33%) | 18 (60.00%) | |||||
| Luminal B1 | 84 | 69 (82.14%) | 55 (65.48%) | |||||
| Luminal B2 | 19 | 14 (73.68%) | 11 (57.89%) | |||||
| HER-2+ | 21 | 16 (76.19%) | 11 (52.38%) | |||||
| Triple-negative | 16 | 12 (75.00%) | 10 (62.50%) | |||||
| NSLN | 20.596 | 45.947 | ||||||
| Metastatic | 74 | 70 (94.59%) | 67 (90.54%) | |||||
| Nonmetastatic | 96 | 63 (65.63%) | 38 (39.58%) | |||||
IDC, International Classification of Diseases; SLN, sentinel lymph node; NSLN, nonsentinel lymph node; VEGF, vascular endothelial growth factor; HER-2, human epidermal growth factor receptor 2.
| Ultrasonic characteristics | n | VEGF-C positive (n = 133) | p | VEGF-D positive (n = 105) | p | |||
| Tumour size | 0.019 | 0.889 | 0.327 | 0.568 | ||||
| 66 | 52 (78.79%) | 39 (59.09%) | ||||||
| 104 | 81 (77.88%) | 66 (63.46%) | ||||||
| Burr | 6.690 | 0.010 | 4.857 | 0.028 | ||||
| No | 97 | 69 (71.13%) | 53 (54.64%) | |||||
| Yes | 73 | 64 (87.67%) | 52 (71.23%) | |||||
| Calcifications | 1.272 | 0.259 | 0.334 | 0.564 | ||||
| No | 78 | 58 (74.36%) | 50 (64.10%) | |||||
| Yes | 92 | 75 (81.52%) | 55 (59.78%) | |||||
| Blood flow signal grade | 4.930 | 0.177 | 4.014 | 0.260 | ||||
| 0 | 11 | 8 (72.73%) | 4 (36.36%) | |||||
| I | 48 | 41 (85.42%) | 33 (68.75%) | |||||
| II | 83 | 66 (79.52%) | 51 (61.45%) | |||||
| III | 28 | 18 (64.29%) | 17 (60.71%) | |||||
| Location | 0.699 | 0.951 | 3.529 | 0.273 | ||||
| Above outside | 59 | 46 (77.97%) | 32 (54.24%) | |||||
| Below outside | 17 | 14 (82.35%) | 12 (70.59%) | |||||
| Inside and above | 10 | 8 (80.00%) | 7 (70.00%) | |||||
| Inside and below | 13 | 11 (84.62%) | 10 (76.92%) | |||||
| Areola region | 71 | 54 (76.06%) | 44 (61.97%) | |||||
VEGF, vascular endothelial growth factor.
NSLN metastases occurred in 74 of 170 patients (74/170, 43.53%), with 307
metastases. The NSLN metastasis rates of VEGF-C- and D-positive patients
(52.63%, 63.81%) were higher than those of negative patients (10.81%, 10.77%)
(p
| Group | n | NSLN Nonmetastatic | NSLN Metastatic | p | ||
| VEGF-C | Positive | 133 | 63 (47.37%) | 70 (52.63%) | 20.596 | |
| Negative | 37 | 33 (89.19%) | 4 (10.81%) | |||
| VEGF-D | Positive | 105 | 38 (36.19%) | 67 (63.81%) | 45.947 | |
| Negative | 65 | 58 (89.23%) | 7 (10.77%) | |||
VEGF, vascular endothelial growth factor; NSLN, nonsentinel lymph node.
There were 70 NSLN metastases in VEGF-C-positive patients (52.63%), and the
number of metastases was 12.03% (16/133), 15.04% (20/133), 7.52% (10/133),
6.02% (8/133), and 13.53% (18/133) for 1, 2, 3, 4, and
 Fig. 2.
Fig. 2.Proportion of positive NSLN in VEGF-C-positive and -negative patients. VEGF, vascular endothelial growth factor; NSLN, nonsentinel lymph node.
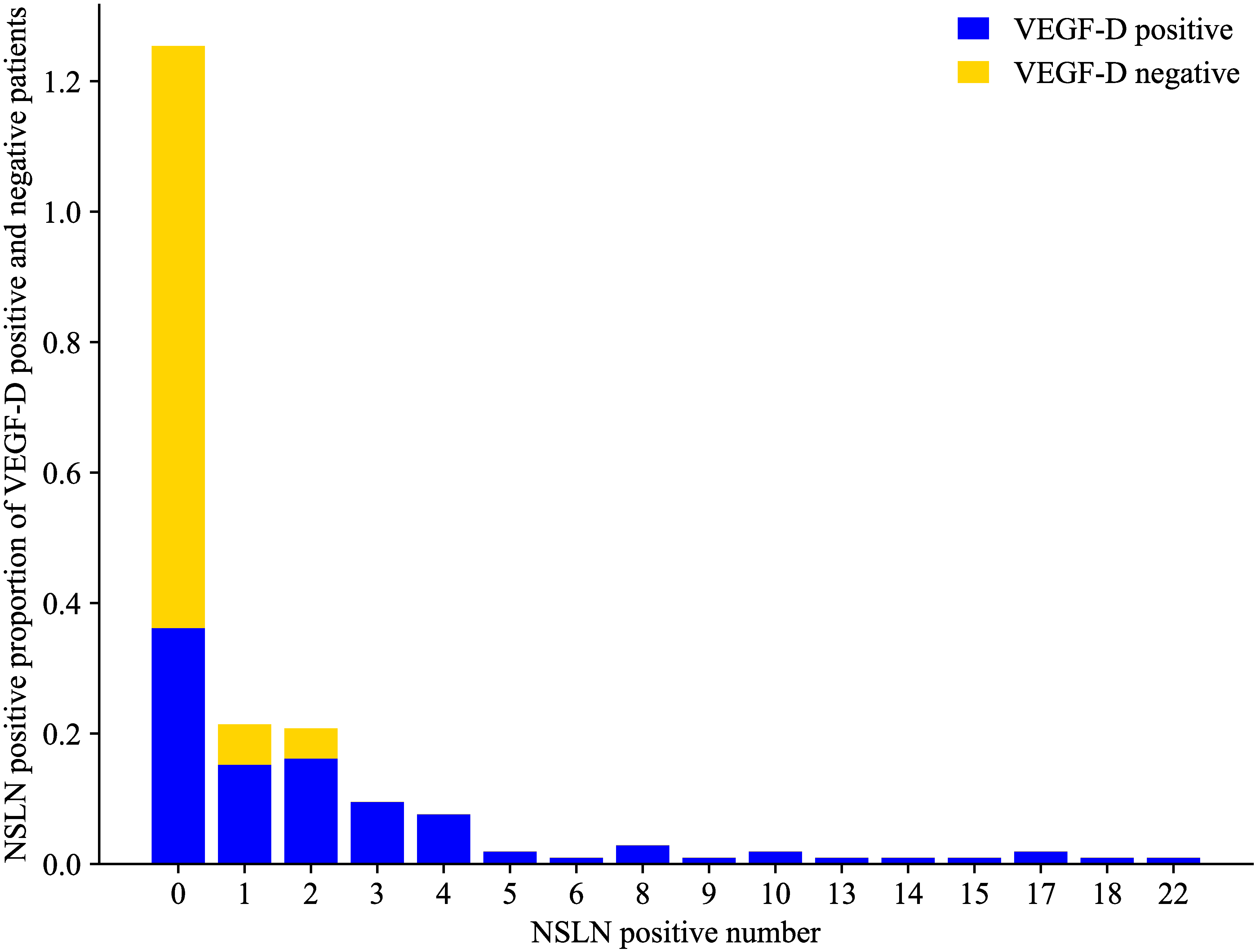 Fig. 3.
Fig. 3.Proportion of NSLN-positive patients among VEGF-D-positive and -negative patients. VEGF, vascular endothelial growth factor; NSLN, nonsentinel lymph node.
A total of 170 SLN-positive patients were taken as samples, and whether
the patients had NLSN metastasis (metastasis = 1, nonmetastasis = 0) was taken as
the dependent variable. The clinicopathological features and ultrasound features
of the patients were used as independent variables to establish a binary logistic
regression analysis model. The stepwise regression method was adopted, with
| Factors | Assignment | SE | Wald |
p | OR | 95% CI | |
| Vascular infiltration | Yes = 1, No = 0 | 1.203 | 0.543 | 4.915 | 0.027 | 3.332 | 1.150~9.654 |
| SLN positive number | Continuous input | 1.681 | 0.532 | 9.975 | 0.002 | 5.372 | 1.892~15.247 |
| SLN positive percentage ¿0.5 | Yes = 1, No = 0 | 1.849 | 0.876 | 4.456 | 0.035 | 6.353 | 1.317~26.874 |
| Burr | Yes = 1, No = 0 | 1.315 | 0.561 | 5.495 | 0.019 | 3.724 | 1.240~11.180 |
| VEGF-C positive | Yes = 1, No = 0 | 1.698 | 0.757 | 5.032 | 0.025 | 5.464 | 1.239~24.091 |
| VEGF-D positive | Yes = 1, No = 0 | 1.527 | 0.624 | 5.997 | 0.014 | 4.604 | 1.356~15.625 |
SLN, sentinel lymph nodes; VEGF, vascular endothelial growth factor; OR, odds ratio; CI, confidence interval; SE, standard error.
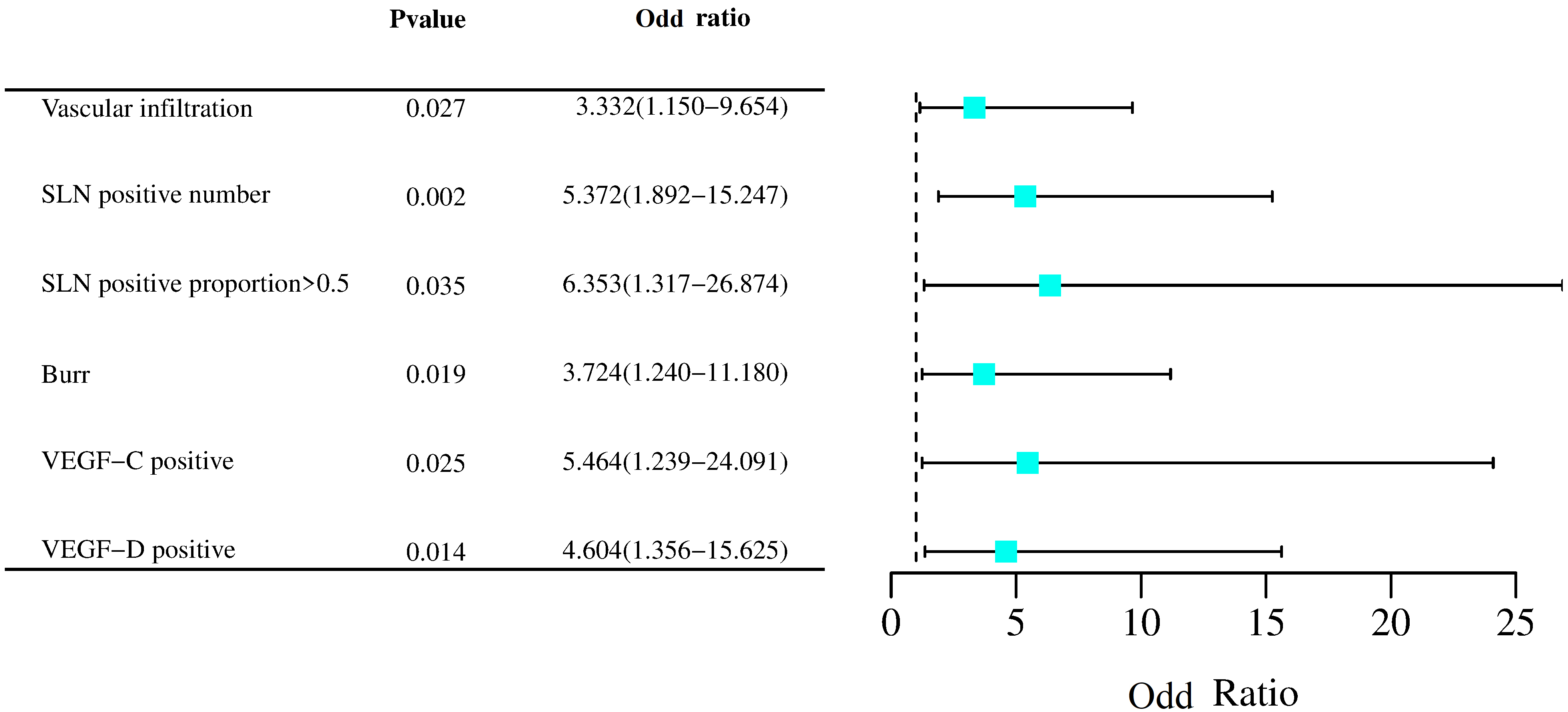 Fig. 4.
Fig. 4.Forest plot of risk factors for NSLN metastasis. SLN, sentinel lymph nodes; VEGF, vascular endothelial growth factor.
ROC diagnostic models were developed with NSLN metastasis as positive and NSLN
nonmetastasis as negative. ROC analysis showed that the AUC (95% CI) of VEGF-C
and VEGF-D applied alone to predict NSLN metastasis was 0.645 (0.568–0.717) and
0.755 (0.683–0.817), respectively, which was lower than the predictive efficacy
of Model 2 (VEGF-C + VEGF-D + Burr) (Z = 6.005, p
| Indicators | Cut-off | Sensitivity% (n/N) | Specificity% (n/N) | Youden | AUC (95% CI) |
| Model 1 | 81.08% (60/74) | 78.12% (75/96) | 0.5921 | 0.859 (0.796~0.907) | |
| Model 2 | 86.49% (64/74) | 65.62% (63/96) | 0.5211 | 0.822 (0.756~0.877) | |
| Model 3 | 90.54% (67/74) | 62.50% (60/96) | 0.5304 | 0.823 (0.757~0.877) | |
| VEGF-C | Positive | 94.59% (70/74) | 34.38% (33/96) | 0.2897 | 0.645 (0.568~0.717) |
| VEGF-D | Positive | 90.54% (67/74) | 60.42% (58/96) | 0.5096 | 0.755 (0.683~0.817) |
Note: The joint application was fitted with the LogP model. Model 1: VEGF-C,
VEGF-D, vascular infiltration, SLN positive number, SLN positive proportion
 Fig. 5.
Fig. 5.ROC curve of the combination of VEGF-C and D ultrasound and pathological features to predict NSLN metastasis. ROC, receiver operating characteristic; VEGF, vascular endothelial growth factor; SLN, sentinel lymph nodes.
The VEGF-C- and D-positive rates in breast cancer tissues were 78.24% and
61.76%, respectively, which were higher than the positive rates in
paraneoplastic tissues (36.47% and 18.82%), indicating that VEGF-C and D in
breast cancer lesions were abnormally highly expressed, while they were expressed
at low levels in paraneoplastic tissues. The VEGF-C- and D-positive rates showed
differences in the presence or absence of vascular infiltration, the number of
positive SLNs
In this study, the logistic analysis revealed that the risk of NSLN metastasis
increased 2.332-fold in those with vascular infiltration compared to those
without vascular infiltration, and the risk of NSLN metastasis increased
4.372-fold for each increase in the number of positive SLNs. SLN positive
percentage
In this study, the NSLN metastasis rate in VEGF-C- and D-positive patients was higher than that in VEGF-negative patients, and logistic analysis showed that VEGF-C- and D-positive patients increased 4.464- and 3.604-fold, respectively, compared with negative patients, indicating that both positive proteins were risk factors for NSLN metastasis. Li et al. [19] concluded that VEGF-C and its receptor 3 can promote lymph node metastasis in renal cell carcinoma. One study noted that VEGF-C and D transcript and protein expression levels were increased in different grades of endometrial cancer [20], suggesting that high expression of both increases the metastatic intensity of endometrial cancer. This result suggested that the possible mechanism by which VEGF-C and D promote lymphatic metastasis is that VEGF-C and D can bind to VEGFR-3 in lymphatic vessel endothelial cells and activate multiple signalling pathways, such as phosphatidylinositol 3-kinase regulatory subunit 1 (PIK3R1) [21] and wingless-type mouse mammary tumor virus (MMTV) integration site family (WNT5A) [22], to promote lymphatic vessel expansion, thus increasing lymph node metastasis risk. In addition, this study showed by ROC analysis that the AUC (95% CI) of VEGF-C and D combined with ultrasound and pathological features to predict NSLN metastasis was 0.859 (0.796–0.907), which was higher than the predictive efficacy of VEGF-C and D alone and their combined ultrasound or pathological features, respectively, indicating that these two proteins alone and in combination with ultrasound and pathological features help to improve the early identification of NSLN metastasis and reduce the risk of unnecessary surgery in people with a low risk of NSLN metastasis.
The analysis of this study as a single-centre retrospective study still has some limitations, the sample size that can be included in this time is limited, and the measurements of VEGF-C and VEGF-D have some differences between different testing institutes, which still needs to be improved in the future work, and can be explored by multicentre prospective large-sample data analysis implementation.
The ultrasound and pathological features of SLN-positive breast cancer, such as
vascular infiltration, a high number of positive SLNs, an SLN positivity ratio
The simulation experiment data used to support the findings of this study are available from the corresponding author upon request.
All the authors have contributed to the document retrieval, conception and design of this study. Material preparation, data collection and analysis, and patient follow-up were conducted by JC, QL, WL, XT, ZW and LX. The first draft of the manuscript was written by JC and QL, and all authors commented on the first few versions of the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of The Third Hospital of Nanchang (approval number: 201526).
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to Shegan Gao and Yiwen Liu for advice on the research concept and design, and Yiwen Liu for professional editing and language revision, and Yiwen Liu, Xiang Yuan, Jinyu Kong, Wei Sun, Yijun Qi, Hong Yang for development of methodology, and Shegan Gao, Fuyou Zhou, Kuisheng Chen, Haijun Yang for acquisition of data, and Jinyu Kong, Yiwen Liu, Wei Sun for analysis and interpretation of data.
This study was supported by the project of Jiangxi Provincial Health Commission (project No. 20164016).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
