- Academic Editor
Background: Caveolin-1 (Cav-1) is known to regulate angiogenesis. However, little is known about Cav-1’s role in polycystic ovary syndrome (PCOS). This study aims to investigate Cav-1’s expression in the endometrium of PCOS rats during the implantation window and its association with endometrial angiogenesis. Methods: Female Sprague Dawley (SD) rats were randomly divided into the control and PCOS groups. The rats in the PCOS group mated after ovulation induction, while the rats in the control group mated during the estrus period. On the 2nd and 5th days of pregnancy, the rats were sacrificed, and the endometrium was isolated from their uteruses. Immunohistochemistry (IHC) staining of CD34 was used to evaluate the endometrial micro-vessel density (MVD). The expression of Cav-1 and vascular endothelial growth factor (VEGF) in the endometrium of both groups was assessed through IHC staining and real-time reverse transcription polymerase chain reaction (RT-PCR) analysis. Results: IHC analysis of endometrium tissue sections showed reduced MVD in PCOS rats on both the 2nd and 5th days of pregnancy. The endometrial expression of Cav-1 and VEGF were also significantly downregulated in the PCOS group compared to the control group during the implantation window. Interestingly, the endometrial expression of Cav-1 was positively correlated with MVD and VEGF. Conclusions: Our study demonstrated the decreased endometrial angiogenesis in PCOS rats during implantation window. This decrease was linked to decreased Cav-1 expression, suggesting Cav-1 is a potential therapeutic target for PCOS patients.
Polycystic ovary syndrome (PCOS) is a dysovulation disorder that affects female reproductive endocrine and metabolic dysfunction, leading to infertility in women [1]. While methods to promote follicle maturation and induce ovulation can improve ovulation rates, PCOS patients still face challenges with low pregnancy and high abortion rates [2, 3]. Previous research has identified that PCOS patients experience issues with endometrial development and embryo receptivity, which contributes to infertility following ovulation promotion [4]. Good endometrial blood perfusion is an important condition for the establishment of endometrial receptivity. Insulin resistance [5, 6], high luteinizing hormone, and high androgen [7, 8] can lead to insufficient uterine blood perfusion in PCOS patients, which negatively impacts endometrial receptivity [9].
Caveolin-1 (Cav-1) is a surface marker protein of plasma membrane vesicles. It is abundantly expressed in endothelial cells, participates in cell proliferation and differentiation, tissue angiogenesis, and signal transduction, and is closely related to angiogenesis [10]. At present, it has been widely reported that Cav-1 regulated tumor growth and angiogenesis following cerebral ischemia, however, its role in endometrial receptivity remains unstudied. This study aims to investigate the Cav-1 expression in the endometrium of PCOS rats during the implantation window period and examine its correlation relationship with endometrial angiogenesis, to discover potential therapeutic targets for improving the endometrial receptivity of PCOS patients.
Twelve virgin female Sprague Dawley (SD) rats weighing about 50
Rats were fed adaptively for three days and randomly divided into the PCOS group (n = 6) and the control group (n = 6). The PCOS group was fed a high-fat pellet feed (Wuxi Fan Bo Biotechnology Co., Ltd, Wuxi, Jinagsu, China). According to Lee MT et al. [11], dehydroepiandrosterone (DHEA, 60 mg/kg) (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 0.2 mL oil injection (Sigma-Aldrich, St. Louis, MO, USA). The rats in the control group were subcutaneously injected with 0.2 mL oil injection on the back of the neck every day, and those in the PCOS group were subcutaneously injected with 0.2 mL DHEA + oil injection on the back of the neck every day for 20 days. Vaginal smears were taken from the 10th day of injection to observe the two estrous cycles. The continuous appearance of keratinocytes indicated the successful establishment of the PCOS model. Afterward, the PCOS group received 0.52 mg/kg letrozole (Jiangsu Hengrui Pharmaceutical Co., LTD, Lianyungang, Jiangsu, China) by gavage, while the control group received 2.1 mL/kg normal saline by gavage. At 04:00 PM on the 2nd day after gavage, female and male SD rats in both groups mated during estrus in a ratio of 1:1. The presence of a vaginal plug the next morning indicated pregnancy.
The rats in both groups were sacrificed on the 2nd and 5th days of pregnancy. Under the aseptic condition, the bilateral uteruses were removed and the endometrium was separated by cutting the uterine horn.
Vaginal smears of rats were obtained and fixed every morning beginning on the 10th day of oil injection, and the changes in the estrous cycle were then analyzed under a microscope (Olympus Corporation, Tokyo, Japan). Furthermore, the rats’ bodyweight was assessed once a week.
Endometrial tissue from rats was extracted and fixed in paraffin on the 2nd and
5th day of pregnancy, as described aboved. The following procedures were used for
immunohistochemistry (IHC) staining: deparaffinization of tissue slides was
followed by antigen retrieval. The slides were incubated with primary antibodies
(as mentioned below), followed by horseradish peroxidase (HRP)-conjugated secondary antibodies (as
mentioned below), and diaminobenzidine (DAB, Abcam, Fremont, CA, USA) after
endogenous peroxidase was blocked with 3% H
The endometrium of the 5th day of pregnancy was harvested and homogenized, and
total RNA was extracted suing Trizol (Invitrogen, Carlsbad, CA, USA). Then, 1
µg of extracted total RNA was reverse transcribed using the Reverse
transcription kit following the manufacturer’s instructions (Ambion Company,
Austin, TX, USA), and 10 ng cDNA was used for PCR amplification
(PrimeScript™ RT reagent Kit, TAKARA, Kusatsu, Shiga, Japan), and mRNA
expression was quantified using the 2
| Primer name | Sequence (5′→3′) |
| TCGTACCACTGGCATTGTGAT | |
| AGGGAGCGCGTAACCC | |
| Cav-1-F | CAACGACGACGTGGTCAAGA |
| Cav-1-R | CATAGGGATGCCGAAGATGGT |
| VEGF-F | CGACAGAAGGGGAGCAGAAAG |
| VEGF-R | AATTGGACGGCAATAGCTGC |
Cav-1, Caveolin-1; VEGF, vascular endothelial growth factor; F, Forward; R, Reverse; RT-PCR, real-time reverse transcription polymerase chain reaction.
Statistical analysis was performed with SPSS (version 20.0; IBM, Armonk, NY,
USA). All data followed normal distributions and were presented as mean
Starting in the fourth week, high-fat feeding induced significant weight gain in the PCOS group rats (Fig. 1). We also observed that rats in the PCOS group exhibited irregular estrous cycles during the estrus period, whereas rats in the control group had regular cycles, indicating the successful establishment of PCOS model (Fig. 2).
 Fig. 1.
Fig. 1.Bodyweight growth trend of the two groups of rats. In the PCOS group, the bodyweight increased significantly from the 4th week of high-fat feeding, and it was more evident than that in the control group at the 6th week. PCOS, polycystic ovary syndrome.
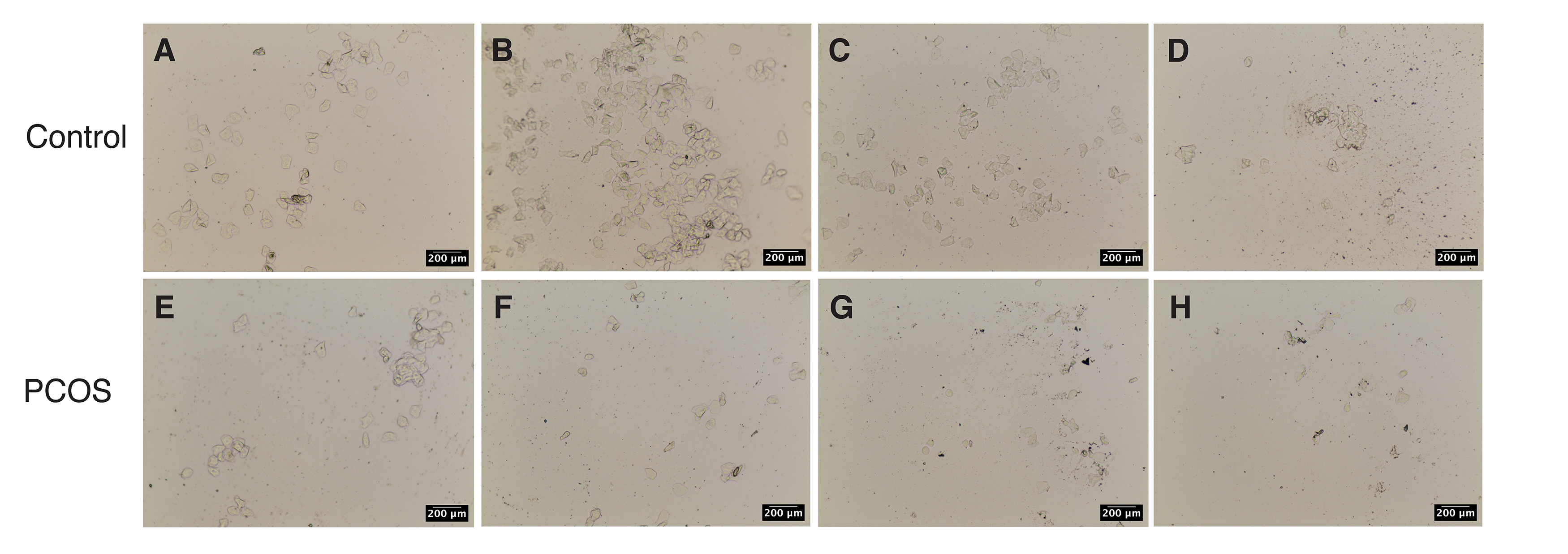 Fig. 2.
Fig. 2.Vaginal smears. (A–D) A normal estrous cycle exists in the control group. (A) Proestrus. (B) Estrous period. (C) Metaestrus. (D) Estrus interval. (E–H) When compared with the control group, the rats in the PCOS group were always in the estrus interval and lost the regular estrous cycle after building.
To evaluate the endometrial blood perfusion during pregnancy, we immunoassayed the endometrium slices with CD34, a microvessel-specific marker. As shown in the figure, on the 2nd and 5th days of pregnancy, the MVD in the PCOS group’s endometrium was significantly lower than in the control group. Furthermore, MVD increased with gestation in both control and PCOS groups, although there was no statistical significance (Fig. 3A–D).
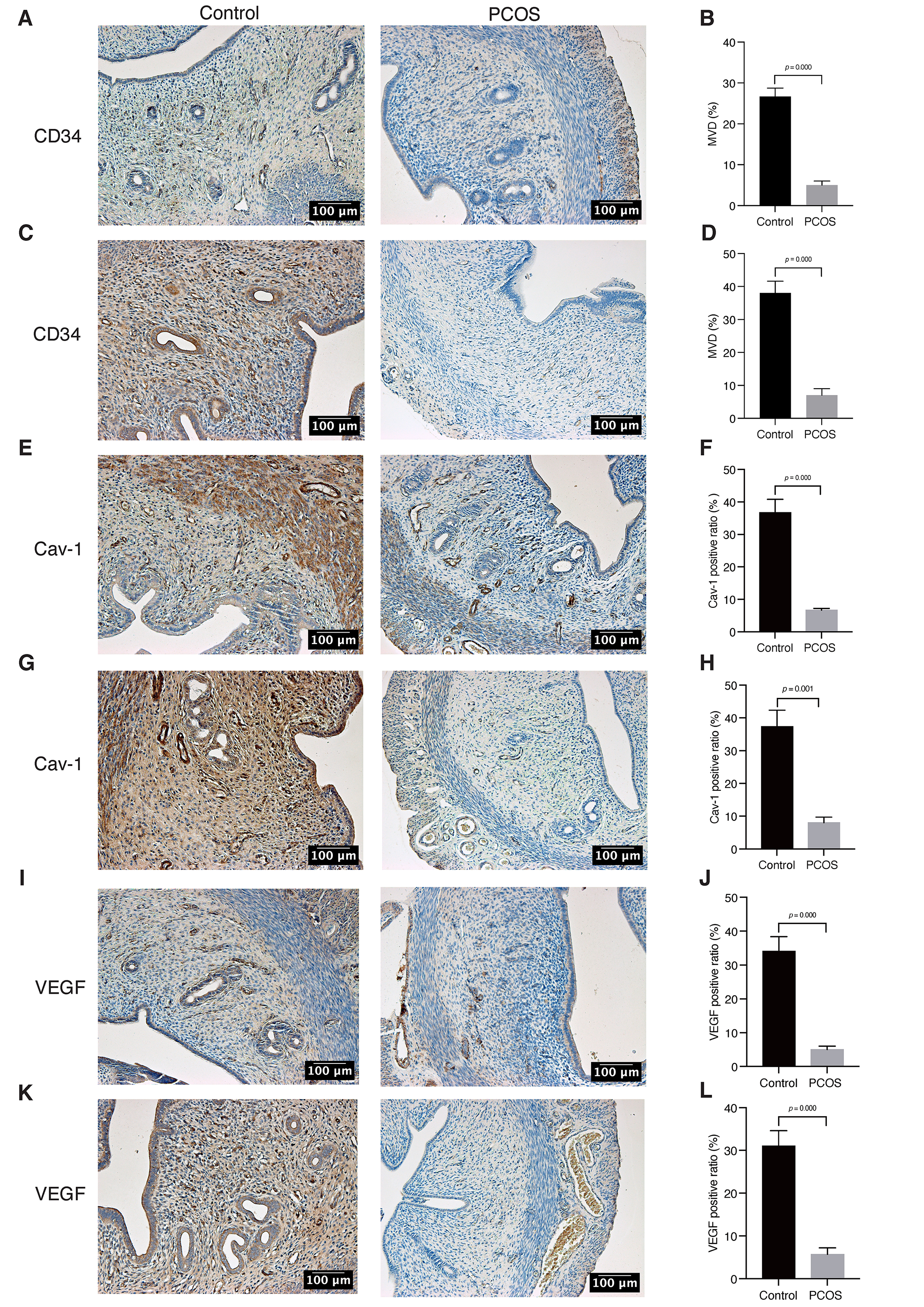 Fig. 3.
Fig. 3.Tissues CD34, Cav-1, and VEGF expression by IHC. (A,B) On the
2nd day of pregnancy, IHC staining for the expression of CD34. (C,D) On the 5th
day of pregnancy, IHC staining for the expression of CD34. (E,F) On the 2nd day
of pregnancy, IHC staining for the expression of Cav-1. (G,H) On the 5th day of
pregnancy, IHC staining for the expression of Cav-1. (I,J) On the 2nd day of
pregnancy, IHC staining for the expression of VEGF. (K,L) On the 5th day of
pregnancy, IHC staining for the expression of VEGF. Original magnification:
200
Cav-1 and VEGF protein were mainly expressed in endometrial stromal and glandular epithelial cells [13, 14]. We utilized immunohistochemical labeling on endometrial slices to investigate the alterations in Cav-1 and VEGF expression in PCOS rat models. Our data revealed that the expression of Cav-1 and VEGF protein in the endometrium of the PCOS group was significantly lower than that of the control group during the implantation window stage. In addition, we observed slightly upregulated expression of VEGF in the PCOS group on the 5th day compared to the 2nd day. In both the control and PCOS groups, the expression of Cav-1 increased over time. However, no statistical significance was seen (Fig. 3E–L). On the 5th day of pregnancy, real-time reverse transcription polymerase chain reaction (RT-PCR) analysis revealed that the expression of Cav-1 and VEGF mRNA in the endometrium of the PCOS group was significantly lower than that of the control group (Fig. 4).
 Fig. 4.
Fig. 4.Tissues Cav-1 and VEGF mRNA expression by real-time reverse transcription polymerase chain reaction (RT-PCR). (A,B) On the 5th day of pregnancy, the expression of Cav-1 and VEGF mRNA in the endometrium of the PCOS group was significantly lower than that in the control group. A significant difference was noted between the PCOS and control groups.
We further explored the association between Cav-1 and endometrial angiogenesis. Interestingly, correlation analysis showed that Cav-1 expression was positively correlated with MVD (r = 0.818, p = 0.047) and VEGF (r= 0.835, p = 0.039) in the endometrium of the PCOS group at the implantation window stage (Fig. 5), suggesting that Cav-1 regulates endometrial angiogenesis.
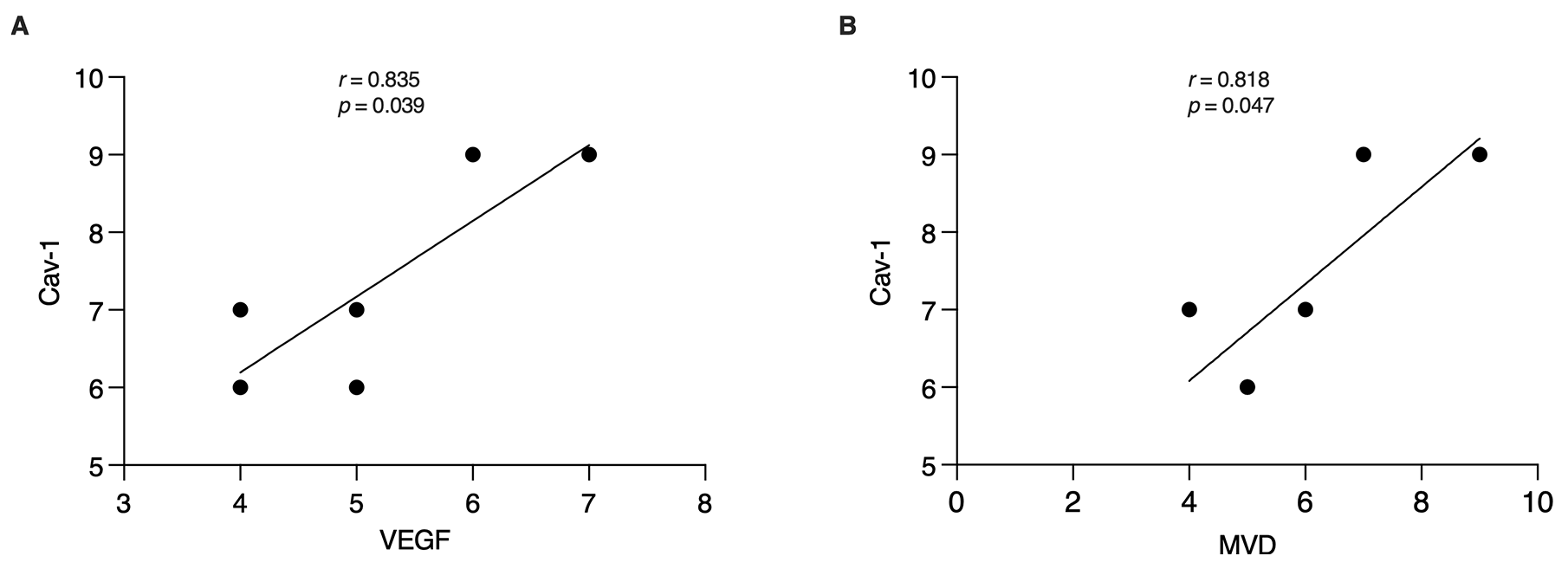 Fig. 5.
Fig. 5.Association between Cav-1 and endometrial angiogenesis. (A,B) According to the correlational analysis, in the PCOS group, Cav-1 was positively correlated with MVD and the VEGF expression. The coefficient of determination of Cav-1 and VEGF was r = 0.835, p = 0.039. The coefficient of determination of Cav-1 and MVD was r = 0.818, p = 0.047.
PCOS is a prevalent metabolic and ovulatory disorder in women of childbearing age, with a prevalence rate as high as 4–15% [15]. According to research, in addition to ovulation abnormalities, poor endometrial receptivity is an important cause of PCOS infertility [16, 17]. Endometrial receptivity is the ability of the endometrium to facilitate embryo implantation during a specified period [18], which necessitates appropriate endometrial blood vessel development. It is also a prerequisite for embryo implantation and pregnancy to proceed successfully [19, 20].
MVD is a crucial parameter for assessing angiogenesis. A greater extent of neovascularization is indicated by a higher MVD in tissues. Endometrial blood flow may therefore be well determined by assessing endometrial MVD by immunostaining endometrial microvascular endothelial cells with CD34 antibody [21]. Our findings revealed an increase in MVD as gestational days progressed (Fig. 3A–D). During the implantation window time, however, the MVD of the endometrium in the PCOS group was significantly lower than in the control group (Fig. 3A–D). This suggests that endometrial angiogenesis was decreased, resulting in insufficient blood flow during the implantation window in PCOS individuals.
VEGF is an important angiogenesis-promoting cytokine. According to research, VEGF primarily affects endothelial cells in endometrial vessels, stimulating their proliferation and migration [22, 23]. Because VEGF expression closely correlates with uterine blood flow, it is a significant marker of endometrial receptivity [24, 25]. Inadequate VEGF levels have been linked to decreased endometrial receptivity, which may potentially impact the development of early villi [26]. Consequently, VEGF plays an important role in endometrial neovascularization [27]. Our findings show that VEGF expression is lower in the endometrium of PCOS rats during the implantation window period (Fig. 3I–L), aligning with the lower levels of endometrial MVD in those rats. These results reinforce the notion that endometrium angiogenesis is impaired during the PCOS implantation window, potentially affecting endometrial receptivity, development, and embryo implantation. However, our data also reveal that VEGF expression is downregulated in the conrol group during gestation, which contradicts the MVD results. Given the small sample size of rats in this experiment, this discrepancy may be due to experimental errors.
Caveolae are vesicle-like structures formed by the invagination of the cytoplasmic membrane [28]. Among these structures, Cav-1 is a crucial structural protein [28] that is highly expressed in vascular endothelial cells [29]. It has been found that Cav-1 participates in the process of angiogenesis and is a key regulator of this process [30]. As a result, it has garnered significant attention in tumor and cerebral ischemia research. Given that the biological behavior of embryo implantation resembles that of tumor invasion and metastasis, the successful implantation of an embryo relies on a well-supplied endometrium. Therefore, Cav-1 may become a new target for investigating endometrial angiogenesis. In this study, we found a significant reduction in Cav-1 expression in the endometrium of the PCOS group compared to the control group (Fig. 3E–H). Furthermore, this reduction was positively correlated with the expression of MVD and VEGF (Fig. 5). Recent study has also confirmed that Cav-1 can influence VEGF expression in umbilical vascular endothelial progenitor cells [31]. After Cav-1 gene knockout, the number of VEGF-mediated blood vessels decreased significantly [32]. Collectively, these findings suggest that the decreased expression of Cav-1 in the endometrium during the PCOS implantation window may be an important reason for the decrease in endometrial angiogenesis.
According to research, the chronic inflammatory process plays an important role
in the pathogenesis of PCOS [33]. Some inflammatory markers, such as tumor
necrosis factor-alpha (TNF-
Our study found that PCOS rats have reduced endometrial angiogenesis during the implantation window. This reduction might be attributed to the decreased Cav-1 expression, indicating that Cav-1 could be a potential therapeutic target for PCOS infertility.
Cav-1, Caveolin-1; PCOS, polycystic ovary syndrome; SD, Sprague Dawley; MVD,
micro-vessel density; IHC, immunohistochemistry; VEGF, vascular endothelial
growth factor; DHEA, dehydroepiandrosterone; TNF-
Data are available upon reasonable request.
HYX contributed to study design, data acquisition, data analysis, and manuscript draft. GZW organized the whole study and rewrote the manuscript. Both authors read and approved the final manuscript. Both authors contributed to editorial changes in the manuscript. Both authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This study was approved by the Clinical Center Laboratory Animal Welfare & Ethics Committee of Shanghai General Hospital (Ethics approval number: 2021AWS0171).
Not applicable.
This research was supported by the Special Project of Traditional Chinese Medicine Scientific Research of Shanghai Health and Family Planning Commission (2016LP024) and “Xinglin Xinxing” Talent Project of Shanghai [ZY( 2018-2020)-RCPY-3006].
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
