1 Endometriosis Research Center, Iran University of Medical Sciences (IUMS), 1416634793 Tehran, Iran
2 Department of Artificial Intelligence, Smart University of Medical Sciences, 1587656811 Tehran, Iran
3 Department of Surgery, Faculty of Medicine, Shahid Beheshti University of Medical Sciences, 1983969411 Tehran, Iran
Abstract
Background: Hysteroscopy is a
pragmatic diagnostic and operative method for the assessment of uterine
pathologies. Cervical preparation is an important step for hysteroscopy, and it
is recommended in order to reduce complications. The efficacy of Dilapan-S and
Misoprostol as two means of cervical preparation were evaluated and compared in
this study. Methods: This randomized clinical trial was conducted on
women referred to Rasoul-e-Akram Hospital outpatient department. A total of 120
menopausal and non-menopausal patients with no history of vaginal delivery were
included in this study. 400 micrograms of misoprostol and Dilapan-S were used for
cervical ripening three hours before hysteroscopy. Cervical dilation was measured
by the diameter of the largest dilator inserted without resistance prior to
hysteroscopy. The time needed to achieve 9 mm dilatation was recorded.
Complications of the procedure were evaluated and compared in both groups.
Results: The size of the largest Hegar dilator without resistance in
Dilapan-S and misoprostol groups were 7.6
Keywords
- Dilapan-S
- cervical ripening
- misoprostol
- hysteroscopy
Nowadays, hysteroscopy is the most widely used procedure by gynecologic surgeons to manage abnormal bleeding, infertility, and abortion [1, 2]. The main associated complication of this exam is the rupture and false tract formation of the cervix as the result of the hysteroscope insertion into the cervix [3]. Cervical preparation via pharmacological interventions or mechanical dilatation has been used to reduce complications [4, 5]. With the increasing prevalence of outpatient hysteroscopy, attention has been paid to the side effects of misoprostol preparation, including cramps, nausea, vomiting, diarrhea, and fever. In various studies on cervical dilatation in labour, the use of mechanical dilators, such as Dilapan-S, has been more acceptable due to the lack of systemic complications [6, 7].
In 1982, a synthetic dilator (Hipan plastic polymer) called Dilapan represented superiority to laminaria in safety, more ergonomic shape, and clinically more dilatation. Dilapan is an osmotic hygroscopic cervical dilator made from a stabilized hydrogel absorbing fluids from the cervical cell, thereby, reduces water in cell membranes along with mechanical dilation. The Food and Drug Administration (FDA) approved the application of Dilapan-S in 2015 for cervical ripening. Studies have shown Dilapan-S safety and effectiveness for cervical dilation in vaginal delivery. Dilapan is a laminaria-like compound that has undergone synthetic modifications. Studies have suggested that not only dose Dilapan-S expand faster than the previous formula but it also has a stronger core to reduce fragmentation. Moreover, it has shown to dilate the cervix, approximately 4–6 times greater than its maximum diameter (3.3 to 6.3 times its dry diameter). Dilapans̕ hygroscopic effect continues for 24 hours [7, 8, 9].
The effectiveness of Dilapan-S in cervical dilation using transvaginal ultrasound has shown that maximum cervical preparation is achieved in the first 6–8 hours after insertion of Dilapan. In 3 hours after insertion, cervical dilatation reached 72.5% and up to the size of 7.9 mm [10]. Previous studies have recommended the use of this dilator at least 12 hours before surgery [4]. However, the use of misoprostol up to three hours before surgery has shown effectivity in cervical preparation [11]. Therefore, in this study, the efficacy and side effects of using Dilapan-S as a mechanical dilator three hours before hysteroscopy were evaluated and compared with misoprostol.
This randomized single-blind controlled trial was conducted on candidates of operative hysteroscopy referred to Rasoul-e Akram hospital clinic of Iran University of Medical Sciences. The study was registered at Iran university of Medical Sciences ethics committee (Number: IR.IUMS.REC.1399.764) and also in Iran Registry of Clinical Trial (IRCT: https://en.irct.ir/, trial registration number: IRCT20191123045476N2).
According to Bartz et al.’s study [12], and using the above formula, the minimum sample size required for each group was 120 people (60 people in each group).
The sample size in this study, taking into account the type 1 error of 5% and power of 90% based on previous studies (5.7 mm in the misoprostol group (SD: 1.6 mm) and 4.7 mm in the control group (SD: 1.5 mm)).
The number of 60 people was determined for each group. This sample size was determined by STATA method (Stata/MP, MP ver.16, StataCorp LLC, College Station, TX, USA)
The inclusion criteria were as follows: non-virgin patients with no recent or current pelvic infection; no history of cervical surgery or confirmed cervical malignancy; no history of vaginal delivery; no contraindications to misoprostol, including asthma, glaucoma, and heart or kidney disease; and no history of misoprostol allergy. The exclusion criteria were severe uterovaginal prolapse preventing the vaginal misoprostol to be inserted, severe cervical stenosis or endocervical canal lesion, and intolerance for Dilapan-S or misoprostol.
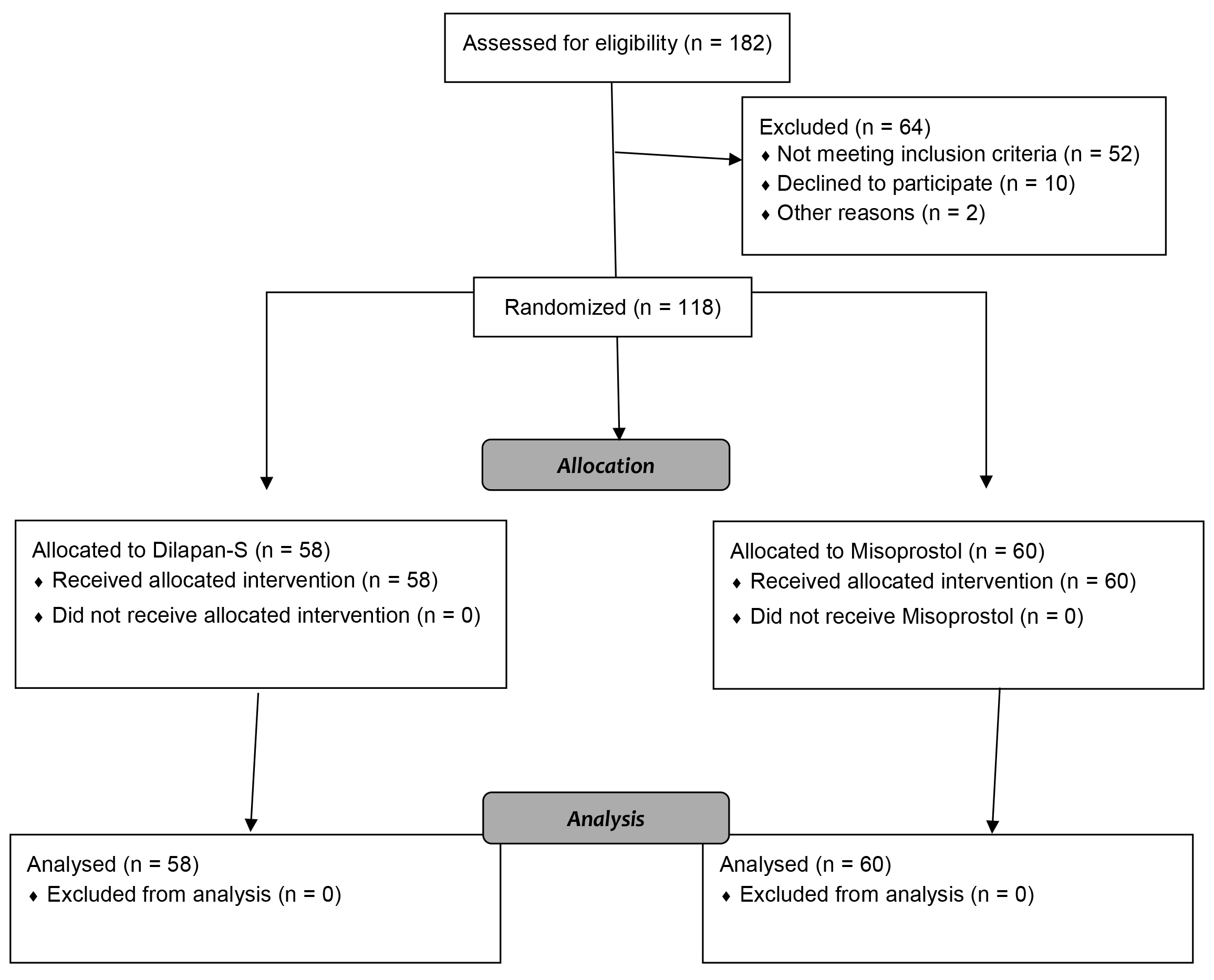
Out of 182 patients, 120 were eligible for the study. From February 2021 to February 2022, a total of 120 menopausal and pre-menopausal patients meeting the criteria were included in this study (the CONSORT Flow Diagram is shown in Fig. 1). After explaining the advantages of the research, obtaining patient informed consent, and recording a complete medical history, the patients were randomly divided into two groups of Dilapan-S and misoprostol, based on computer-generated admission numbers. In the first group, 400 mg of misoprostol (Misotec, Aburaihan, Tehran, Iran) was inserted vaginally in the posterior fornix three hours before surgery in a lithotomy position. In the second group, Dilapan-S (Kardanexir, Tehran, Iran) was implanted in the cervical canal three hours prior to the surgery in a lithotomy position so that it would pass through the cervical external and internal os; the Dilapan-S rod handle was at the level of the cervical external os.
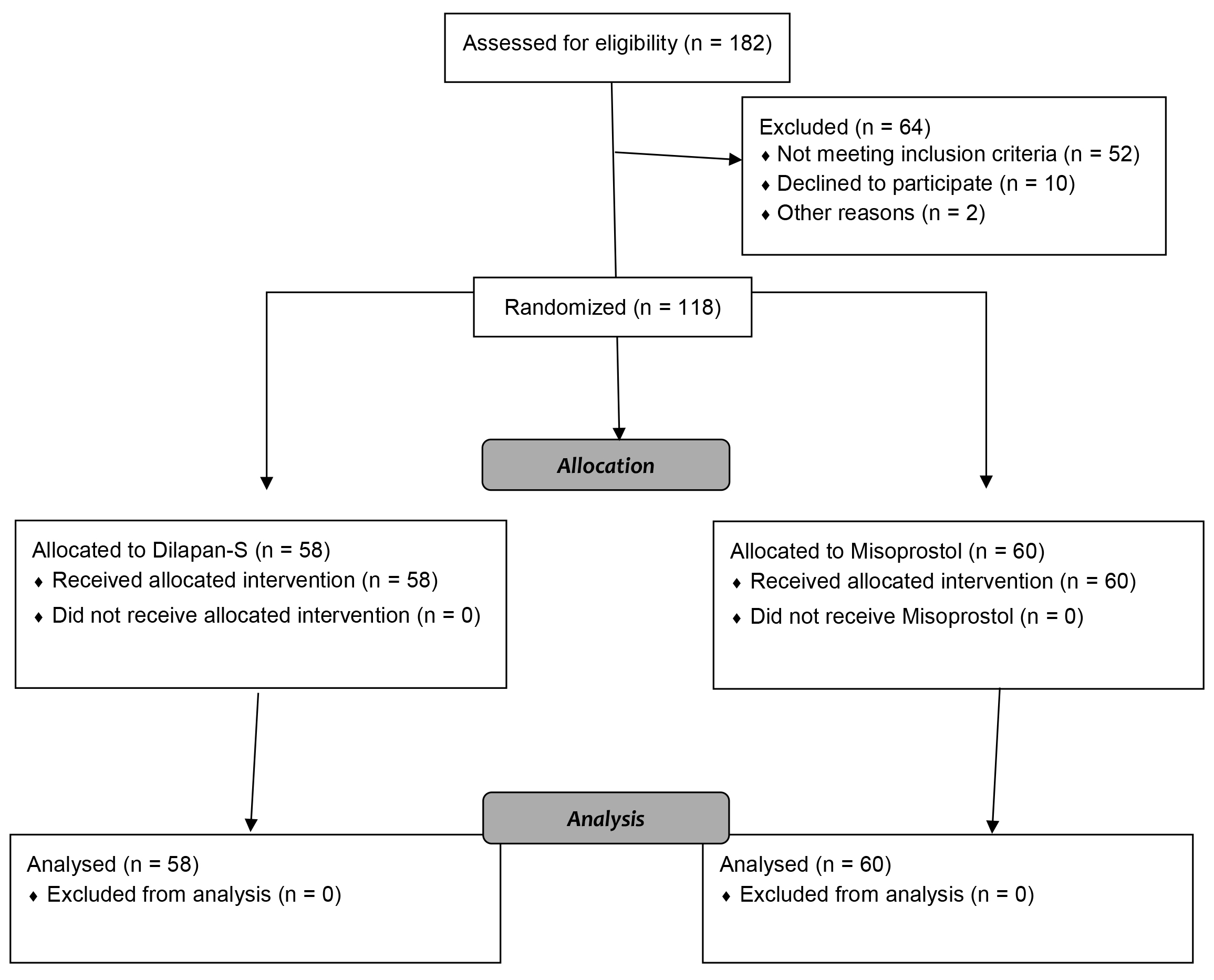 Fig. 1.
Fig. 1.CONSORT flow diagram.
Before the patient’s transfer to the operating room, the side effect of intervention, including abdominal cramps, vaginal bleeding, palpitation, nausea, and headache were investigated, and the patients’ responses were recorded. Surgery was performed under general anesthesia in both groups. Protocol of anaesthesia induction was standard for all patients and included midazolam 25 µg/kg, fentanyl 2 µg/kg, as premedication, and propofol 2 mg/kg, atracurium 5 mg/kg and lidocaine 1.5 mg/kg. Anaesthesia maintenance was done by isoflurane 1% with control of hemodynamic symptoms.
Dilapan-S and misoprostol residues were removed from the vagina prior to surgery, so the surgeon was blind. To reduce inter-rater reliability, surgery was performed for all the patients by one gynecological surgeon.
After prepping and draping, the surgeon performed a bimanual exam. The uterine position and cervical softness were also examined. The passage of the cervical dilator began with a dilator size of 2 mm. The diameter of the largest dilator inserted without resistance was determined before hysteroscopy. Time to achieve 9 mm dilatation was recorded; the ultimate goal was dilatation with dilator 9 (9 mm). Surgery was performed with a 26-F (9 mm) resectoscope (Karl Storz, Tuttlingen, Germany) with a 12 degree lens. The main outcome was cervical dilation width measured by diameter of largest dilator entered without resistance. Misoprostol side effects, hysteroscopic complications, including rupture of the cervix, false tract formation, and uterine perforation were also examined.
The severity of pelvic pain was measured and recorded two hours after hysteroscopy, based on a 10-point visual analog scale (VAS).
Data were analyzed by SPSS version 26 (IBM Corp., Armonk, NY, USA). T-test was used to compare numerical data between the two groups, the Chi-square test was used for qualitative data, and Kruskal-Wallis test was carried out for ordinal data. The significance level was set at 0.05.
In this study, 120 patients were examined in two groups. Mean of age in
Dilapan-S and misoprostol were 40.5
| Variables | Misoprostol group | Dilapan-S group | p-value | |
| Age (years) | 40.5 |
39.7 |
0.6 | |
| BMI (kg/m |
26.35 |
27.25 |
0.35 | |
| Gravidity | 2 |
2 |
0.79 | |
| Parity | 1 |
1 |
0.73 | |
| Number of years since having the last child | 16 |
14 |
0.58 | |
| Menopause | ||||
| Yes | 5 (8.3) | 4 (6.9) | 0.52 | |
| No | 55 (91.7) | 54 (93.1) | ||
| Cause of hysteroscopy | ||||
| Polyp | 26 (43.3) | 22 (37.9) | 0.9 | |
| Abnormal bleeding | 18 (30) | 18 (31) | ||
| Submucosal myoma | 11 (18.3) | 11 (19) | ||
| Postmenopausal bleeding | 2 (3.3) | 3 (5.2) | ||
| Endometrial thickening | 3 (5) | 4 (6.9) |
BMI, body mass index.
The most common pathological finding was polyps in both groups, and the findings were not different (p-value = 0.6) in two groups (Table 2).
| Final pathology | Misoprostol group | Dilapan-S group | p-value |
| Polyp | 25 (41.7) | 29 (50) | 0.6 |
| Myoma | 11 (18.3) | 10 (17.2) | |
| Hyperplastic endometrium | 24 (40) | 19 (32.8) |
The mean size of the largest dilator inserted without resistance was higher in
Dilapan-S group, 7.6
In the Dilapan-S group, it took significantly less time (p-value
| Groups | Dilapan-S | Misoprostol | p-value | ||
| n | Mean |
n | Mean | ||
| Non-Menopause | 54 | 40 |
55 | 73.9 |
|
| Menopause | 4 | 42.7 |
5 | 84 |
|
| Non-Menopause and Menopause | 58 | 40.2 |
60 | 74.7 |
|
SD, Standard Deviation.
There was a significant difference between the two groups in terms of pain
intensity after surgery (p-value
Preoperative complications were significantly different in both groups (p-value = 0.013). Headache and nausea were not reported by any patients in Dilapan-S group, while they were reported by 4 and 14 patients of misoprostol group, respectively (Table 4).
| Variables | Dilapan-S, n (%) | Misoprostol, n (%) | p-value |
| Abdominal cramps | 3 (37.5) | 29 (53.7) | 0.013 |
| Nausea | 0 (0) | 14 (25.9%) | |
| Headache | 0 (0) | 4 (7.4) | |
| Palpitation | 2 (25) | 4 (7.4) | |
| Vaginal bleeding | 3 (37.5) | 3 (5.6) | |
| Cervical tearing | 0 (0) | 6 (10%) | 0.013 |
| False tract | 2 (3%) | 3 (5%) | 0.6 |
| Tenaculum site bleeding | 1 (1.7%) | 27 (45%) | |
| Uterin perforation | 0 (0) | 0 (0) | - |
Regarding the intraoperative complications in both groups, cervical tearing was
detected in six cases (10%) in the misoprostol group, while it was not reported
in the Dilapan-S group (p-value
In this study, 60 patients in the misoprostol group and 58 patients in the Dilapan-S group were examined and the outcomes and complications were reported. The side effects and intra-operative complications of hysteroscopy were significantly better in the Dilapan-S group compared to the misoprostol group. The preoperative side effects of the drug and the postoperative side effects of surgery were significantly lower in the Dilapan-S group. For instance, abdominal cramps and nausea were more common in the misoprostol group with rates of 53.7% and 25.9% versus 37.5% and 0%, in the Dilapan group.
As regards to some characteristics of Dilapan-S compared to other mechanical dilator such as Laminaria, Dilapan-S is superior in its ergonomic shape, safety and also fast acting [7].
In this study, the use of Dilapan-S three hours before surgery provided the desired preparation for hysteroscopy, without causing remarkable complications or discomforts in patients. Similarly, in a study by Yu et al. [4], the first clinical trial in this field, the osmotic dilator caused cervical ripening more effectively than misoprostol. In their study, Dilapan-S was used 12 hours before surgery [4]. In similar studies, the efficacy time was estimated to be 3–24 hours for misoprostol and 2–4 hours for Dilapan-S as an osmotic dilator [13, 14, 15]. In this study, the use of these two methods three hours before surgery was sufficiently effective in cervical preparation. In contrast to previous reports [16], in this study, at the time of Dilapan-S withdrawal, we did not encounter any Dilapan-S residues or injury to the uterus in any patients.
Cervical injury during hysteroscopy can be related to the dilatation of the cervix prior to the hysteroscopy. It is known that Dilapan-S swells by absorbing water from the environment and ripens the cervix both chemically and physically through application of radial force during swelling. This external pressure releases F-prostaglandins and shows clinical efficacy within three to 24 hours. It seems that the cause of reduced bleeding at the site of the tenaculum in the Dilapan-S group is related to this mechanism.
Based on the findings of this study, the short-term use of Dilapan-S for cervical preparation was significantly better than misoprostol. Uterine rupture is one of the most important complications of hysteroscopic therapy, as reported in 1% of cases in previous studies [17], which can be reduced by cervical preparation. Furthermore, no side effects of prostaglandins, such as cramps and nausea, were detected in the Dilapan-S group in our study. Although using an effective ripening method is crucial for safe hysteroscopy, importance of adequate hysteroscopic training should be emphasized [18].
The results of the present study showed that the short-term use of Dilapan-S before surgery, due to proper preparation of the cervix, increases the chance of successful hysteroscopy, without causing intolerance or major complications in patients or necessitating a longer preoperative hospital stay. This could be the method of choice, especially when there is sensitivity to prostaglandins. In addition, in therapeutic hysteroscopies that require more maneuvers, the osmotic dilator is more effective and associated with less bleeding. However, one of the advantages of using misoprostol is its cheaper price and availability, and the patient can easily place it herself.
The Advantages of our study is using ripening agent which easily can be used at the short time before hysteroscopy, so operative hysteroscopy needed cervical ripening could be performed without longer hospital stay preoperatively.
The limitation of our study is the small number of menopausal women. Further studies are suggested in larger group to more precisely determine the outcomes and complications. In future studies, it is highly recommended to survey Dilapan-S and misoprostol in menopausal woman.
The datasets used and analyzes could be available from the corresponding author on reasonable request.
RS: Conceptualization, data collection, manuscript writing, patients treatment and follow-up; SC: Conceptualization, manuscript writing, patients treatment and follow-up; KT: Conceptualization, manuscript revision, patients treatment and follow-up; LH: Conceptualization, manuscript revision, patients treatment and follow-up; RD: Conceptualization, manuscript writing, patients treatment and follow-up; BS: Study design, data analysis, manuscript revision; SR: Conceptualization, supervision, manuscript revision, patients treatment and follow-up. All authors made a significant contribution to the work, and have approved the final version.
The study was registered in Iran University of Medical Sciences ethics committee (Number: IR.IUMS.REC.1399.764) and also in Iran Registry of Clinical Trial (IRCT; trial registration number: IRCT20191123045476N2). Written informed consent was obtained from all patients after explaining the purpose and procedure of the study.
We would like to thank Rasoul-e Akram Hospital of Iran University of Medical Sciences.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.