- Academic Editor
Background: The adverse perinatal outcome caused by adenomyosis has
been widely concerned recently, but little attention has been paid to whether the
positional relationship between placenta and adenomyotic lesion influences the
maternal and perinatal outcomes. Methods: A total of 311 women with
adenomyosis who were pregnant greater than 20 weeks gestation and delivered at
Women’s Hospital, Zhejiang University School of Medicine between January 2010 and
December 2021 were recruited. The positional relationships between placenta and
adenomyotic lesions were determined. The patients were divided into two
subgroups: group 1, placenta located on or above the adenomyotic lesion; and
group 2, placenta located far away from the adenomyotic lesion. The clinical data
of two groups were retrospectively analyzed. Results: We found a higher
rate of diffuse adenomyosis (62.65% vs. 46.21%, p = 0.01),
coexisting endometriosis (31.93% vs. 15.86%, p = 0.002),
preterm delivery (34.94% vs.15.17%, p
Adenomyosis, an estrogen-dependent chronic inflammatory gynecological benign disease, is defined as the presence of endometrial glands and stroma within the myometrium of the uterus, resulting in dysmenorrhea and infertility [1, 2, 3]. The exact pathogenesis of adenomyosis remains unclear, although the incidence of adenomyosis tends to occur in younger women and is rising [4, 5, 6]. Although there are many mechanisms to explain infertility caused by adenomyosis, such as abnormal endometrial receptivity and oviduct peristalsis, it is still not clear whether infertility is the result or the cause of adenomyosis [6, 7, 8, 9]. Recently, as more women delay their first pregnancy, adenomyosis, like endometriosis, is attracting more attention because of its increasing impact on fertility and pregnancy outcomes [10, 11, 12, 13, 14, 15, 16, 17]. Consequently, it is necessary to identify the high risk factors affecting the fertility and reproductive outcomes of patients with adenomyosis prior to pregnancy in order to minimize obstetric complications.
Increasing evidence from recent studies has demonstrated that many factors,
including age, uterine size, disease severity, subtype and concomitant diseases,
can affect the pregnancy outcomes of women with adenomyosis [18, 19, 20, 21, 22, 23, 24]. One study of
uterus-sparing surgery for patients with adenomyosis by Kishi et al.
[18] showed that the clinical pregnancy rate of women
Many obstetric complications in pregnant women with adenomyosis, such as preeclampsia, preterm birth, premature rupture of membrane, small for gestational age, malpresentation and abruption have received more attention in recent years [25, 26, 27, 28]. More recently, a study by Ono et al. [29] of the positional relationship between the placenta and the adenomyosis lesion influenced the perinatal outcomes demonstrating that placental localization near or above adenomyotic lesions increased the risk of perinatal complications. Based on previous research, we performed a retrospective study [30], and found that our results were consistent with those reported by Ono et al. [29]. There was a significant increase in the obstetric morbidity and lower birth weight when the placenta overlaid an adenomyosis lesion. If women with severe diffuse adenomyosis do not have normal myometrium, their placenta will be implanted on the adenomyotic lesions. This may be the cause of infertility or pregnancy failure in patients with severe diffuse adenomyosis.
We investigated if the positional relationship between placenta and adenomyotic lesions could be identified in pregnant women with adenomyosis, and all patients were divided into two subgroups (Group 1: placenta on or above the adenomyotic lesion; Group 2: placenta far away from the adenomyotic lesion) according to the relationship between placental implantation site and the adenomyotic lesion. A comparative analysis was performed so as to clarify the influence of the relationship between placental implantation site and adenomyosis lesion on perinatal complications.
Between January 2010 and December 2021, a total of 688 pregnant women with adenomyosis who delivered at Women’s Hospital, School of Medicine, Zhejiang University were recruited for this study. The clinical data of all pregnant women with adenomyosis, including age, gravidity, parity, history of surgery, hormone therapy, adenomyosis subtype, gestational age, natural pregnancy, assisted reproductive technology (ART) pregnancy, comorbidity, pregnancy induced hypertension (PIH), gestational diabetes mellitus (GDM), fetal growth restriction (FGR), placental abnormalities, premature rupture of fetal membranes (PROM), preterm birth, mode of delivery, size of the placenta, neonatal birth weight, and postpartum hemorrhage were retrospectively obtained and recorded from the original electronic medical record (EMR) of hospitalized pregnant women with adenomyosis. This study was approved by the Ethics Committee of Women’s Hospital, School of Medicine, Zhejiang University (No. IRB-20210310-R). All patients were exempt from informed consent because this study was retrospective.
Of the 688 pregnant women with adenomyosis, 332 were excluded because there were
no nuchal translucency (NT) ultrasound records during 11–13
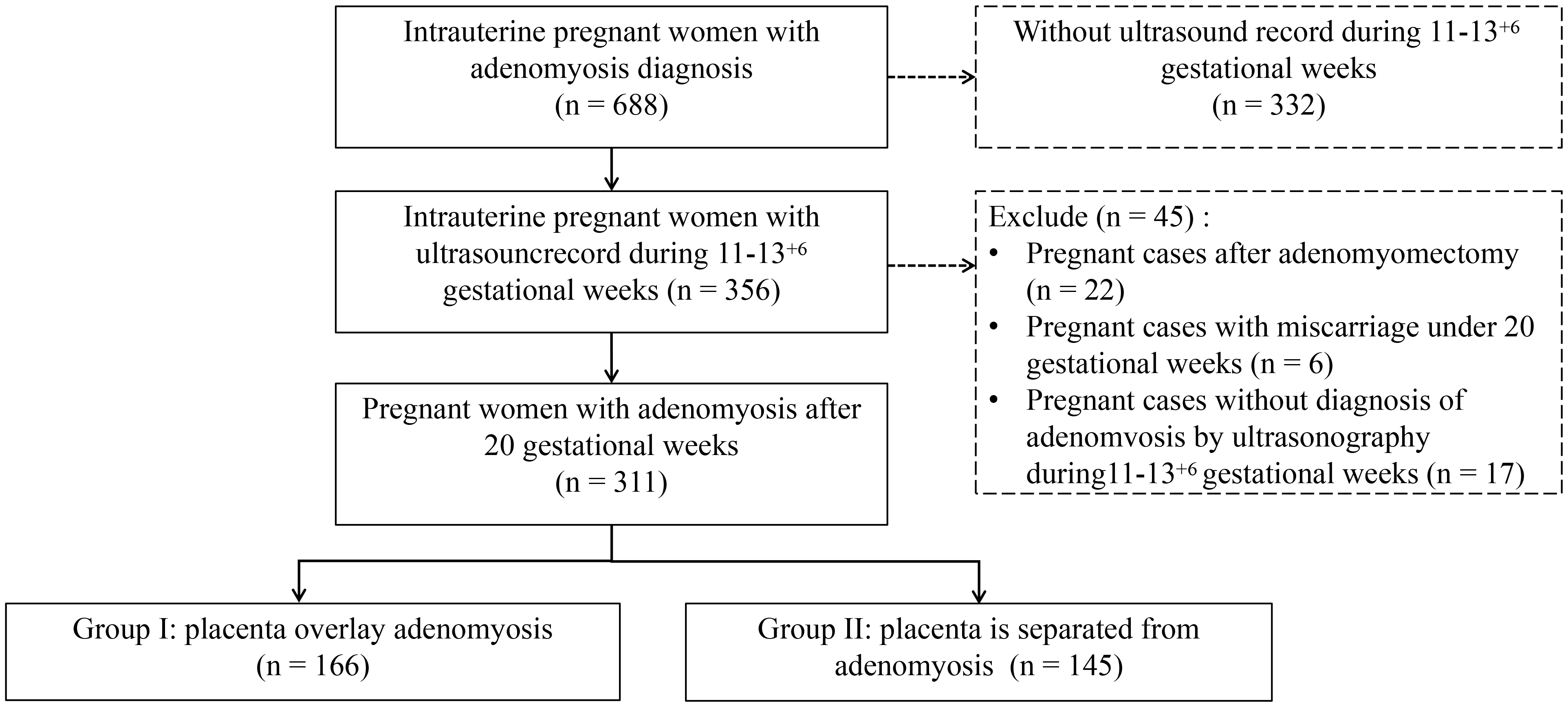 Fig. 1.
Fig. 1.Flow chart of patient inclusion.
 Fig. 2.
Fig. 2.The diagram of the positional relationships between placenta and adenomyotic lesion. Group I: the placenta was located on the adenomyotic lesion (as shown in (a,b)). Group II: the placenta was far away from the adenomyotic lesion (c,d).
In order to avoid deviation, two experienced ultrasound experts who were not involved in the research re-examined the ultrasound images to confirm the diagnosis of adenomyosis and the positional relationship between placenta and adenomyotic lesion. The ultrasonic diagnostic criteria for adenomyosis were formulated according to the 2022 consensus on the revised definition of morphological ultrasound assessment (MUSA) features of adenomyosis [31].
Summary statistics were used to characterize the study population and
differences between groups were assessed using Chi-square or Fisher’s exact test
for categorical variables, Student’s T test for normally distributed
continuous variables and Mann–Whitney U tests for non-normally distributed data.
Multiple logistic regression analyses were used to determine the association
between factors and preterm birth if the placenta was located above adenomyosis.
Covariates in the multivariate models were selected based on a significant
association at alpha
In this study, the patients were screened as shown in Fig. 1. The final study
included 311 pregnant women with adenomyosis. The mean age was 34.39
| Overall (n = 311) | Group I (n = 166) | Group II (n = 145) | p | ||
| Age (mean (SD)) | 34.39 (4.53) | 34.09 (4.36) | 34.72 (4.70) | 0.22 | |
| Age group (%) | |||||
| 156 (50.16) | 86 (51.81) | 70 (48.28) | 0.61 | ||
| 155 (49.84) | 80 (48.19) | 75 (51.72) | |||
| Parity (%) | |||||
| 0 | 123 (39.55) | 62 (37.35) | 61 (42.07) | 0.46 | |
| 188 (60.45) | 104 (62.65) | 84 (57.93) | |||
| ART (%) | 69 (22.19) | 40 (24.10) | 29 (20.0) | 0.47 | |
| Adenomyosis type (%) | |||||
| Focal | 140 (45.02) | 62 (37.35) | 78 (53.79) | 0.005 | |
| Diffuse | 171 (54.98) | 104 (62.65) | 67 (46.21) | ||
| Pre-pregnant hysterauxesis (%)* | 41 (18.30) | 21 (18.58) | 20 (18.02) | 1.00 | |
| Scarred uterus (%) | 133 (42.77) | 63 (37.95) | 70 (48.28) | 0.09 | |
| History of endometriosis (%) | 76 (24.44) | 53 (31.93) | 23 (15.86) | 0.002 | |
SD, standard deviation; ART, assisted reproductive technology.
*Pre-pregnant uterine enlargement was defined as the volume of uterus larger than 3 months of gestation; there were 87 women without the data of pre-pregnancy uterus volume.
All pregnant women were divided into two groups: Group I (n = 166) indicated that the placenta was located on the adenomyotic lesion (as shown in Fig. 2a,b); Group II (n = 145) exhibited that the placenta was far away from the adenomyotic lesion (Fig. 2c,d). Compared with group II, diffuse adenomyosis was more common in group I (104 (62.65%) vs. 67 (46.21%), p = 0.01), as well as more likely to have concordant endometriosis (53 (31.93%) vs. 23 (15.86%), p = 0.002). Considering the age, parity, ART, cases with scarred uterus, history of hormone treatment and pre-pregnancy uterine size, there were no significant differences between group I and group II.
The pre-pregnant uterine size of patients with diffuse adenomyosis was larger than the size of patients with focal adenomyosis in both two groups (the proportion of pre-pregnant uterine size larger than three months gestation, focal vs. diffuse: group I 3 (3/62, 4.84%) vs. 18 (18/104, 17.31%), p = 0.01; group II 2 (2/78, 2.65%) vs. 18 (18/67, 26.87%)).
The results of comparison of perinatal outcomes are detailed in Table 2. Of the
311 cases, 12 (3.86%) cases were diagnosed with cervical incompetence, 45
(14.47%) cases were complicated with PIH, 60 (19.29%) cases were complicated
with GDM, and 15 (4.82%) cases were complicated with oligohydramnios. Twenty
three (7.40%) cases of FGR and 47 (15.11%) cases of premature rupture of fetal
membranes (PROM) were detected. There were 2 cases diagnosed with uterine rupture
or threatened uterine rupture with both cases being in group I. The overall rate
of preterm delivery was 25.72% (80/311), which was significantly higher in group
I than group II (34.94% (58/166) vs. 15.17% (22/145), p
| Overall (n = 311) | Group I (n = 166) | Group II (n = 145) | p | |
| Cervical incompetence (%) | 12 (3.86) | 7 (4.22) | 5 (3.45) | 0.96 |
| PIH (%) | 45 (14.47) | 20 (12.05) | 25 (17.24) | 0.26 |
| GDM (%) | 60 (19.29) | 29 (17.45) | 31 (21.38) | 0.47 |
| Oligohydramnios (%) | 15 (4.82) | 8 (4.82) | 7 (4.83) | 1.00 |
| FGR | 23 (7.40) | 15 (9.04) | 8 (5.52) | 0.33 |
| PROM (%) | 47 (15.11) | 23 (13.86) | 24 (16.55) | 0.62 |
| Uterine rupture or threatened uterine rupture (%) | 2 (0.64) | 2 (1.20) | 0 (0.00) | 0.54 |
| Preterm delivery (%) | 80 (25.72) | 58 (34.94) | 22 (15.17) | |
| Fetal presentation (%) | ||||
| Cephalic | 301 (96.78) | 159 (95.78) | 142 (97.93) | 0.45 |
| Non-cephalic | 10 (3.22) | 7 (4.22) | 3 (2.07) | |
| 5 min Apgar |
11 (3.54) | 9 (5.42) | 2 (1.38) | 0.11 |
| Delivery mode (%) | ||||
| Vaginal delivery | 61 (19.61) | 36 (21.69) | 25 (17.24) | 0.40 |
| Cesarean delivery | 250 (80.39) | 130 (78.31) | 120 (82.76) | |
| Birth weight (median (IQR)) | 3090 (2580, 3400) | 3050 (2350, 3335) | 3100 (2800, 3415) | 0.02 |
| Placenta malposition (%)* | 43 (13.83) | 33 (19.88) | 10 (6.90) | 0.002 |
| Placental abruption (%) | 11 (3.54) | 6 (3.61) | 5 (3.45) | 1.00 |
| Placental surface area (median (IQR)) | 324 (288, 360) | 323 (256, 360) | 342 (306, 360) | 0.004 |
| Blood loss during delivery (median (IQR)) | 300 (200, 400) | 300 (200, 400) | 300 (200, 400) | 0.40 |
| Postpartum hemorrhage | 23 (7.40) | 15 (9.04) | 8 (5.52) | 0.33 |
| Composite neonatal adverse outcomes (%) | 99 (31.83) | 70 (42.17) | 29 (20.00) | |
| Composite maternal adverse outcomes (%) | 124 (39.87) | 65 (39.16) | 59 (40.69) | 0.87 |
PIH, pregnancy induced hypertension syndrome; GDM, gestational diabetes mellitus; FGR, fetal growth restriction; PROM, premature rupture of fetal membranes; IQR, interquartile range.
*Placental malposition includes placenta previa or low-lying placenta.
Neonatal adverse outcomes including: preterm delivery, FGR and 5 min Apgar
Maternal adverse outcomes including: PIH, GDM, uterine rupture or threatened uterine rupture, placental abruption and postpartum hemorrhage.
 Fig. 3.
Fig. 3.Spearman correlation analysis showed that the birth weight was positively associated with placental surface area.
There were no significant differences for cervical incompetence, PIH, GDM, oligohydramnios, FGR, PROM, uterine rupture or threatened uterine rupture, fetal presentation, fetal distress, delivery mode, placental abruption and blood loss during delivery between group I and group II.
As shown in Table 3, 5 variables were found to be associated with composite
neonatal adverse outcome by multivariable logistic regression analysis. Placenta
location above the uterine adenomyosis increased the risk of composite neonatal
adverse outcome (odds ratio (OR): 3.13, 95% confidence interval (95% CI): 1.47–7.00, p = 0.004). There were 4
other risk factors related to composite neonatal adverse outcome, including PIH
(OR: 3.46, 95% CI: 1.39–8.70, p = 0.01), placenta malposition (OR:
3.28, 95% CI: 1.18–9.22, p = 0.01), placenta abruption (OR: 10.14,
95% CI: 2.26–54.44, p = 0.003) and scarred uterus (OR: 2.71, 95% CI:
1.17–6.46, p = 0.02). Moreover, searman correlation analysis
demonstrated that the birth weight was positively associated with placental
surface area (R = 0.44, p
| OR | 95% CI | p value | |||
| (Intercept) | 0.03 | 0.01 | 0.14 | ||
| Group | |||||
| I (n = 166) | 3.13 | 1.47 | 7 | 0.004 | |
| II (n = 145) | Reference | ||||
| ART | |||||
| yes (n = 69) | 1.44 | 0.55 | 3.67 | 0.45 | |
| no (n = 242) | Reference | ||||
| Age | |||||
| 1.33 | 0.62 | 2.88 | 0.47 | ||
| Reference | |||||
| Parity | |||||
| Multipara (n = 188) | 1.5 | 0.58 | 3.96 | 0.4 | |
| Primipara (n = 123) | Reference | ||||
| Adenomyosis type | |||||
| Diffuse (n = 171) | 1.54 | 0.7 | 3.41 | 0.29 | |
| Focal (n = 140) | Reference | ||||
| Pre-pregnant enlarged uterus | |||||
| yes (n = 41) | 0.85 | 0.31 | 2.19 | 0.74 | |
| no (n = 270) | Reference | ||||
| History of endometriosis | |||||
| yes (n = 76) | 1.25 | 0.53 | 2.88 | 0.6 | |
| no (n = 235) | Reference | ||||
| Cervical incompetence | |||||
| yes (n = 12) | 1.71 | 0.18 | 20.46 | 0.65 | |
| no (n = 299) | Reference | ||||
| PIH | |||||
| yes (n = 45) | 3.46 | 1.39 | 8.7 | 0.01 | |
| no (n = 266) | Reference | ||||
| GDM | |||||
| yes (n = 60) | 0.97 | 0.35 | 2.43 | 0.95 | |
| no (n = 251) | Reference | ||||
| Placenta malposition* | |||||
| yes (n = 43) | 3.28 | 1.18 | 9.22 | 0.02 | |
| no (n = 268) | Reference | ||||
| Placental abruption | |||||
| yes (n = 11) | 10.14 | 2.26 | 54.44 | 0.003 | |
| no (n = 300) | Reference | ||||
| Oligohydramnios | |||||
| yes (n = 15) | 1.96 | 0.23 | 12.22 | 0.49 | |
| no (n = 296) | Reference | ||||
| PROM | |||||
| yes (n = 47) | 2.41 | 0.86 | 6.52 | 0.09 | |
| no (n = 264) | Reference | ||||
| Delivery mode | |||||
| Cesarean delivery (n = 250) | 0.73 | 0.25 | 2.2 | 0.56 | |
| Vaginal delivery (n = 61) | Reference | ||||
| Scarred uterus | |||||
| yes (n = 133) | 2.71 | 1.17 | 6.46 | 0.02 | |
| no (n = 178) | Reference | ||||
OR, Odds ratio; CI, confidence interval.
*Placental malposition includes placenta previa or low-lying placenta.
Neonatal adverse outcomes includes preterm delivery, FGR and 5 min Apgar
As shown in Table 4, placenta location above the uterine adenomyosis showed no significant effect on the risk of composite maternal adverse outcomes; however, the maternal age older than 35 years might increase the risk (OR: 2.03, 95% CI: 1.11–3.76, p = 0.02).
| OR | 95% CI | p value | |||
| Intercept | 0.46 | 0.15 | 1.36 | 0.17 | |
| Group | |||||
| I (n = 166) | 0.63 | 0.35 | 1.14 | 0.13 | |
| II (n = 145) | Reference | ||||
| ART | |||||
| yes (n = 69) | 0.62 | 0.28 | 1.34 | 0.23 | |
| no (n = 242) | Reference | ||||
| Age | |||||
| 2.03 | 1.11 | 3.76 | 0.02 | ||
| Reference | |||||
| Parity | |||||
| Multipara (n = 188) | 1.47 | 0.7 | 3.12 | 0.31 | |
| Primipara (n = 123) | Reference | ||||
| Adenomyosis type | |||||
| Diffuse (n = 171) | 1.73 | 0.91 | 3.32 | 0.1 | |
| Focal (n = 140) | Reference | ||||
| Pre-pregnant enlarged uterus | |||||
| yes (n = 41) | 0.59 | 0.26 | 1.28 | 0.19 | |
| no (n = 270) | Reference | ||||
| History of endometriosis | |||||
| yes (n = 76) | 0.64 | 0.3 | 1.32 | 0.23 | |
| no (n = 235) | Reference | ||||
| Cervical incompetence | |||||
| yes (n = 12) | 3.34 | 0.48 | 28.61 | 0.22 | |
| no (n = 299) | Reference | ||||
| Placenta malposition* | |||||
| yes (n = 43) | 2 | 0.78 | 5.27 | 0.15 | |
| no (n = 268) | Reference | ||||
| Oligohydramnios | |||||
| yes (n = 15) | 0.6 | 0.08 | 3.09 | 0.56 | |
| no (n = 296) | Reference | ||||
| PROM | |||||
| yes (n = 47) | 0.58 | 0.24 | 1.36 | 0.22 | |
| no (n = 264) | Reference | ||||
| Delivery mode | |||||
| Cesarean delivery (n = 250) | 1.1 | 0.47 | 2.65 | 0.82 | |
| Vaginal delivery (n = 61) | Reference | ||||
| Scarred uterus | |||||
| yes (n = 133) | 0.79 | 0.4 | 1.53 | 0.49 | |
| no (n = 178) | Reference | ||||
*Placental malposition includes placenta previa or low-lying placenta.
Maternal adverse outcomes includes PIH, GDM, uterine rupture or threatened uterine rupture, placental abruption and postpartum hemorrhage.
As shown in Table 5, 3 variables were found to be associated with placenta location above the uterine adenomyosis by multivariable logistic regression analysis. Diffuse adenomyosis (OR: 1.72, 95% CI: 1.08–2.74, p = 0.02), endometriosis history (OR: 2.07, 95% CI: 1.18–3.71, p = 0.01) and placenta malposition (OR: 2.63, 95% CI: 1.25–5.92, p = 0.01) might increase the risk of placenta implantation site overlapping the uterine adenomyosis.
| OR | 95% CI | p value | |||
| Intercept | 0.64 | 0.45 | 0.91 | 0.01 | |
| Adenomyosis type | |||||
| Diffuse (n = 171) | 1.72 | 1.08 | 2.74 | 0.02 | |
| Focal (n = 140) | Reference | ||||
| History of endometriosis | |||||
| yes (n = 76) | 2.07 | 1.18 | 3.71 | 0.01 | |
| no (n = 235) | Reference | ||||
| Placenta malposition* | |||||
| yes (n = 43) | 2.63 | 1.25 | 5.92 | 0.01 | |
| no (n = 268) | Reference | ||||
*Placental malposition includes placenta previa or low-lying placenta.
Recent publications have confirmed that adenomyosis was associated with increased risks of early miscarriage, second trimester miscarriage, preterm delivery, preeclampsia, FGR, placental malposition, postpartum bleeding, cesarean section rate and the incidence of pregnancy by ART [33, 34, 35]. In addition, the gestational age and neonatal birth weight at delivery were significantly lower than those of pregnant patients without adenomyosis and the placental attachment on the area of adenomyotic lesion could be associated with FGR [13, 25].
Our study drew similar conclusions and reconfirmed the conclusion that the relationship between placental implantation site and adenomyotic lesion location had a substantial impact on pregnancy outcome of adenomyosis patients [29]. In our study, the placenta implantation site above the uterine adenomyosis had a high correlation with preterm delivery, lower birth weight and placenta malposition. However, the incidence of FGR showed no significant difference.
Adenomyosis-associated lower neonatal birth weight and preterm delivery were thought to have a pathophysiological correlation with inflammation, free radicals and junctional zone alterations. Specifically, thickening of the myometrial junctional zone was the typical change noted in adenomyosis patients by magnetic resonance imaging (MRI). Placental dysplasia caused by impaired remodeling of spiral arteries in this area creates a hostile environment for the placenta that impedes adequate fetal exchange with the maternal blood supply, possibly through a vascular steal mechanism, leading to numerous adverse pregnancy complications [16, 36, 37, 38, 39]. Previous studies have suggested that fetuses with lower placental weight and smaller placental surface area were at higher risk of developing FGR [40, 41], a finding in accordance with the results of our study that birth weight was positively associated with placental surface area. The lower birth weight in women with placenta location overlapping adenomyosis may be related to the smaller placenta being caused by the adenomyosis.
Adenomyosis is also an independent risk factor for impaired reproductive
function [42]. These patients have a higher incidence of infertility and the
clinical pregnancy rate of assisted reproductive technology in adenomyosis
patients is significantly reduced [36]. In our study, 69 cases (22.19%)
underwent ART, with the percentage being much higher when compared with average
women. Moreover, researchers have found that the adverse pregnancy outcome of
patients whose pregnancy was complicated with adenomyosis was closely related to
the severity of the disease and whether it was diffuse adenomyosis [43, 44]. A
clinical trial from Japan recruited 272 pregnant women with adenomyosis and
reported that the rates of miscarriage (
Although it is currently believed that the adverse pregnancy outcomes of
adenomyosis patients are related to the severity and lesion type of adenomyosis
[43, 44], the exact mechanism leading to the phenomenon is still unclear.
Thickening of the myometrium and endometrial-myometrial junction, elevated
inflammatory cytokines, such as IL-6 and TNF-
Our study retrospectively analyzed the clinical data of 311 pregnant patients complicated with adenomyosis. Our data demonstrated that the placental implantation site was closely related to pregnancy outcomes. If the placenta was located on or close to the adenomyotic lesion, the rate of preterm delivery and placental malposition were significantly increased, and the gestational age, neonatal birth weight and placental size were significantly decreased, which were consistent with the results reported in the literature [13, 53]. Thus, it was further confirmed that there were severe adverse pregnant outcomes in adenomyosis patients whose placenta was closely implanted to the lesion.
Our study detected that diffuse adenomyosis, endometriosis history and placenta malposition might increase the risk of placenta implantation site overlapping the uterine adenomyosis, this pathological mechanism needs to be further explored. This indicates that patients with adenomyosis are recommended to evaluate the severity and lesion type of adenomyosis before pregnancy, and offer early intervention, especially for those women co-existing with an endometriosis history. Once the patient is pregnant, ultrasound and other imaging techniques such as MRI should be utilized to identify the relationship between the embryo implantation site and the lesion position of adenomyosis, and to check the uterine artery pulsation index, in order to closely monitor and intervene when appropriate. An early intervention is the usage of aspirin, in order to prevent early placental dysplasia, including PIH [54] and recurrent miscarriage [55]. The study of Yamanaka [56] revealed that adenomyosis had a risk of activating the blood coagulation system and increased the risk of thrombosis, suggesting that aspirin be considered to be useful in pregnancy complicated by adenomyosis.
Our study determined that severe adverse pregnant outcomes in adenomyosis patients were closely related to the placenta implantation site if it was overlapping lesion. These conclusions are consistent with those reported by Ono et al. [29]. Due to the difficulty to distinguish junctional zone (JZ) in the pregnant uterus by ultrasound screening, it is hard for us to determine the relationship between adenomyosis lesion and JZ, as well as the exact distance between the placenta and the lesions. Thus, our recommendation is that pregnant patients complicated with adenomyosis should undergo MRI in late pregnancy to identify the relationship between placental implantation site and lesion location, uterine artery pulsation index and fetal growth.
In our study, there was no significant difference in the incidence of FGR and PIH, regardless of the location of the placenta. Other researchers have found that PIH was a maternal complication related to an autoimmune mechanism, so it might have something to do with immune pathogenesis of adenomyosis [57]. Although other literature reported that the incidence of FGR in pregnant patients complicated with adenomyosis was mainly related to diffuse adenomyosis [52], our data showed no difference, which might due to one of the limitations of the study (the relatively small number of cases and imperfect retrospective data). There are still other limitations. The history of cesarean section and ART may be associated with the risk of maternal and neonatal outcomes but these cases were not excluded from the study. Fortunately, the patients with the history of cesarean section or ART were symmetrically distributed in two groups. Furthermore, all patients merely had ultrasound images without MRI images since our study was a retrospective approach, so the specific data of the distance between the placental implantation site and the lesion of adenomyosis could not be acquired. Obviously, it is necessary to further comprehensively apply ultrasound and MRI to classify the types of adenomyosis, measure the size of uterus, evaluate the severity of the disease, measure the distance between the placental implantation site and the lesion, and implement a multicenter prospective study with a large sample size to evaluate the adverse pregnancy outcomes of pregnant patients complicated with adenomyosis.
Pregnant women whose pregnancy is complicated by adenomyosis are associated with adverse pregnancy outcomes. When the placental implantation site overlaps the adenomyotic lesion, adverse pregnancy outcomes are more likely to occur, and include preterm delivery and lower birth weight. Women with diffuse adenomyosis, endometriosis history and placenta malposition are more likely to have placental implantation site overlapping the adenomyotic lesion. Therefore, patients with adenomyosis need detailed and thorough evaluation prior to pregnancy. Imaging examinations should be performed during the pregnant process to determine the placental implantation site, uterine artery pulsation index and fetal growth. Close monitoring and early intervention such as consultant-led care when clinically appropriate should be carried out to improve pregnancy outcomes.
The data and materials generated during and analyzed during the present study are available from the corresponding author upon reasonable request.
XZ and PX designed the research study. PX, XH, YZ, YW and GZ performed the research. PX, XH, JW analyzed the data. PX, JW and XZ wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This study was approved by the Ethics Committee of Women’s Hospital, School of Medicine, Zhejiang University (No. IRB-20210310-R). All patients were exempt from informed consent because this study was retrospective.
We appreciate the staff at the Zhejiang University, women hospital for their diligent clinical work and precise data recording in the cases we reported in this article. We thank all the participants in the study.
This study was funded by National Key R&D Program of China (Grant number: 2022YFC2704003) and Zhejiang Provincial Natural Science Foundation of China (grant No. LY23H040005).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
