- Academic Editor
Background: Dysmenorrhea is among the most common conditions among
young women. Herbal medicine is one of the alternative ways to treat
dysmenorrhea. The purpose of this study was to determine the effect of Rosa
Foetida extract along with self-care behaviors on primary dysmenorrhea.
Methods: A randomized clinical trial was conducted on 135 students
residing in dormitories. The subjects were aged 18 to 24 years and unmarried.
They divided into three groups of 45. The students received self-care behavior
training on dysmenorrhea. After the training, two of the three groups received
medications; one with Rosa Foetida extract and another with placebo capsules. The
physical appearance of the capsules was similar. The third group received no
medication. Data were collected through questionnaires including demographic
characteristics questionnaire, a visual analogue scale (VAS), menstrual distress
scale questionnaire (MDQ), dysmenorrhea self-care behaviors scale questionnaire
(DSCS), and Pictorial Blood Loss Assessment Chart (PBAC). Repeated measurement of
analysis of variance (ANOVA) was performed using SPSS software version 22 (IBM
Corp., Armonk, NY, USA) to determine and compare the effects of interventions on
menstrual pain and distress severity. Results: Comparison of the mean
pain intensity before and after intervention implied a reduction in pain;
especially in the Rosa Foetida extract group (p
Dysmenorrhea or menstrual cramps are called painful periods that occur before and during menstruation [1]. It has been reported that 25–85% of women of reproductive age suffer from dysmenorrhea [2]. The most common symptom of dysmenorrhea is menstrual pain due to menstrual prostaglandins release. Other symptoms include fatigue, headache, vomiting, nausea, and dizziness, which can lead to relative unproductivity [3, 4]. Therefore, there has recently been more focus on dysmenorrhea from a social and economic point of view. Dysmenorrhea was also reported to be the most common reason women miss school or work [5].
Dysmenorrhea costs the US workforce 600 million hours and over $2 billion annually [6]. Similar studies report the same in Japan, where over $4.2 billion is lost annually due to dysmenorrhea [7]. It can hence be concluded that dysmenorrhea is one of the most important problems studied worldwide [8, 9].
Methods of dysmenorrhea management include pharmacological and non-pharmacological treatment through complementary and alternative therapies. The drugs used most often to treat dysmenorrhea include synthetic anti-prostaglandins and nonsteroidal anti-inflammatory drugs [10]. Various non-pharmacological approaches have also been proposed, including transcutaneous electrical nerve stimulation (TENS) [11], massage [12, 13], exercise [14], acupressure [15], Yoga [16], aromatherapy [17, 18], herbs such as cumin, dill, and ginger [19], as well as placebos [20]. These treatments have also been shown to be effective in soothing menstrual cramps.
The Persian Yellow Rose, also known as Rosa Foetida, is an edible flower. It belongs to the family of Rosacea. The chemical compounds found in Rosa Foetida are similar to those found in other roses. The Australian Gene Technology Regulatory Authority has reported that roses and their products are not toxic in any way [21, 22]. Traditional treatments also use red rose to treat chest pain, upset stomach, differed blood circulation, and digestive disorders [23, 24].
Modern research on Rosa Damascena report antimicrobial, antimicrobial, anti-inflammatory, analgesic, antioxidant, cancer prevention, antiviral, antiepileptic, antidepressant, relaxing, and sedative effects of Rosa Foetida [24]. Iran’s ecology supports the growth the Rosa family, especially Rosa Foetida. The western and central regions of Iran, as well as the southern regions, are said to have significant rose cultivation. However, there are no clinical studies on the effects of Rosa Foetida on menstrual pain. Therefore, it has been decided to study Rosa Foetida’s influence on menstrual pain and menstrual distress.
This trial was performed according to the guidelines of the Helsinki Declaration and approved by the Ethical Committee of Research, Babol University of Medical Sciences (IR.MUBABOL.REC.1397.059), and recorded at the Iranian registry of clinical trials (IRCT20190318043086N1 registration date: 14/6/2019). The study protocol was fully explained to the participants, who were asked to fill out and sign a written informed consent form prior to their participation. The protocol of this study was published in the Trial journal with https://doi.org/10.1186/s13063-022-06583-4 in August 2022.
This study was a randomized clinical trial was conducted in Iran. The design of
this randomized clinical trial is based on standards devised by the Consolidated
Standards of Reporting Trials (CONSORT) (Fig. 1). Study population consisted of
female students residing at the dormitories of Babol University of Medical
Sciences, Babol, Iran. Eligible dysmenorrheic females were recruited to the
study. Inclusion criteria were unmarried female students residing at the
dormitory with moderate to severe primary dysmenorrhea complaint who met the
inclusion criteria such as: Students who met the diagnostic criteria of Primary
dysmenorrhea [20] with menstrual pain in the last three months, Visual Analog
Scale (VAS) for pain
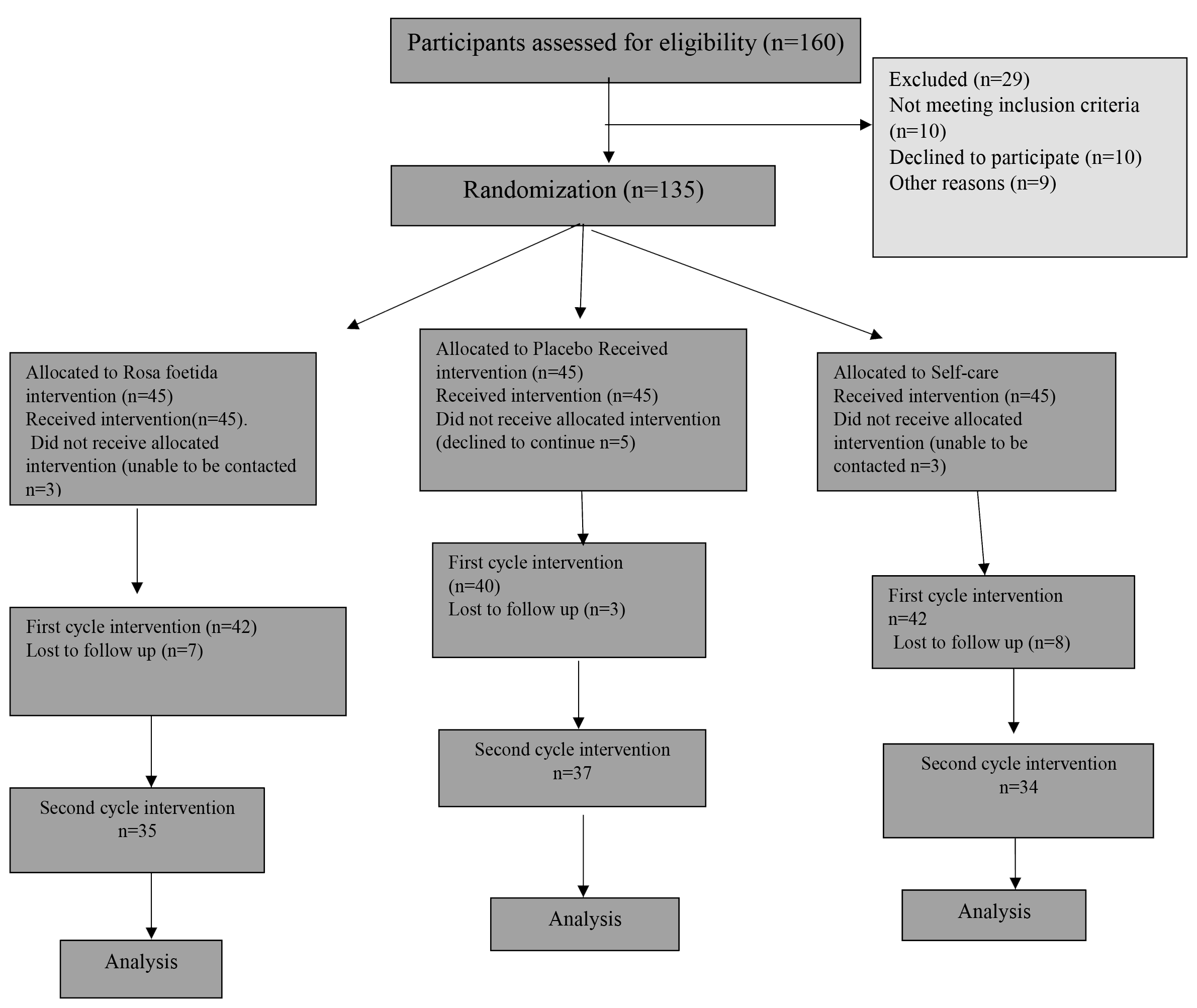 Fig. 1.
Fig. 1.Consolidated Standards of Reporting Trials (CONSORT) flow diagram.
A block-randomized allocation method with 6 blocks was used to assign the eligible subject to three distinct groups [25]. All randomizations were performed using the random website (http://random.org). The randomization table was managed by an individual out of the research team of the trial.
Following the completion of a baseline evaluation, all eligible participants were assigned envelopes randomly (https://random.org). The researcher handing out the envelope was not informed of the content of each envelope. For the sample size estimation, we employed similar number of subjects from other studies with comparable populations [26]. Sample size was estimated to be 135, after considering pain reduction of 1.5 units, variance of 1.8 with 95% confidence interval (95% CI) and 80% Power, and 10% dropouts. The protocol of the study has already been published in the BMC trial Journal [27].
The primary outcome of the study was menstrual pain intensity and the secondary outcomes were menstrual distress, menstrual blood flow, side effects as well as absenteeism frequency. Data were collected using self-report instruments before and at 1, 2, 4, 8, 12, 24 as well as 48 hours after the start of intervention. Moreover, they filled out the Menstrual Distress Questionnaire (MDQ) and Pictorial Blood Loss Assessment Chart (PBAC) at the end of the second day of the menstrual cycle.
The participants were allowed to use standard medication for pain relief if the treatment was not sufficient. The number of the capsules which participants had to take was considered and compared in three groups. The researcher requested them to note the number of the capsules they had to take for relief.
Data collection was performed by questionnaires and scales:
(1) The demographic characteristics questionnaire was used to obtain conventional demographic and reproductive characteristics, including age, menarche age, menstrual bleeding period, and menstrual cycle length.
(2) The Visual Analogue Scale. The Validity and reliability of the VAS was proven by retrospective studies and the Alpha Cronbach of 0.85 was reported by Bani et al. [26] and Williamson et al. [28].
(3) Menstrual Distress Questionnaire. The reliability of the Persian translation of the MDQ was reported to be 0.93 [29].
(4) Dysmenorrhea Self-Care Scale (DSCS) is a modified version of a research instrument proposed by Ching-Hsing et al. [30] which was validated by Kabirian et al. [31]. Similar to the previous questionnaires, the reliability of this method was also proven and the Alpha Cronbach was reported to be 0.78 [30, 32].
(5) Pictorial Blood Loss Assessment Chart: A self-administered pictorial chart recording the number of sanitary pads and/or tampons used according to the degree to which individual items are soiled with blood, blood clots passage, and episodes of flooding. The pictorial chart also proven to be reliable and was validated with an Alpha Cronbach 0.84 [33, 34].
Firstly, dried Rosa Foetida petals were grinned to a fine powder. Then, the powder was added to a decanter along with 70% ethanol solution for 3–4 hours, and the solution was distilled after 24 hours. The distillation process was repeated three times and the resultant extract was added to a rotary device to maximize the concentration of the extract. Finally, the extract was prepared in the form of a powder which was encapsulated for use. The plant extraction and mechanized preparation of capsules was done in the Iranian Institute of Medicinal Plants, Karaj, Iran. The chemical analysis of the extract and the method of the analysis have been presented as a Supplementary File (Supplementary 1).
Intervention consisted of two parts: dysmenorrhea self-care and medication (Fig. 2).
 Fig. 2.
Fig. 2.Sequential diagram of intervention.
Educational classes were held in four sessions (60 to 90 minutes each) during two days. All students who met the criteria for entering and consented to participate in the study during the cycle prior to the medicinal interventions participated in the sessions.
Educational materials for all the groups were the same and were developed based on the menstruation self-care educational booklets provided by the Iranian Ministry of Health. The content of classes is as follows: anatomy and physiology of the reproductive system, healthy diet during menstruation, isometric exercises and hygiene tips during menstruation (bathing, how to properly wash the perineum), relaxation techniques (calm tone and music therapy and breathing), and heat and massage therapy.
The medication consisted of: 200 mg capsules of Rosa Foetida extracts. Retrospective studies reported that the effective herbal dose of damascena is 5 g per day when consumed as dried powder [26, 35], while only 15% of the extract offers the effects, suggesting the effective dose of the extract to be 750 mg. Based on the previous studies mentioned above, we decided to use 600 mg of Rosa extract every day during the interventional days.
The present study prescribes the intake of Rosa Foetida capsules with 200 mg of extract, once every 8 hours.
The placebo was capsules containing 200 mg of starch, produced by the Iranian Institute of Medicinal Plants.
Intervention was performed on the first two days of two consecutive menstrual cycles, and the researcher requested the participants to consume a capsule once every 8 hours as soon as the pain started (usually at the beginning of menstruation or on the first day) and then, record their pain on the VAS at the designated times (1, 2, 4, 8, 12, 24 as well as 48 hours after the start of intervention). At the end of the second day of interventions their menstrual distress and bleeding were assessed by MDQ and PBAC.
Group A received capsules with 200 mg of extract for three daily intakes during initial days of menstruation, in addition to the instructions from the classes.
Group B received capsules loaded with 200 mg of Starch (as the placebo group), in addition to instructions from the classes.
Group C received only instructions from the classes.
The statistical analysis was performed using SPSS Version 22 (IBM Corp., Armonk, NY, USA). The mean pain intensity comparison of the three groups was performed using repeated measurement and analysis of variance (ANOVA) in three different time intervals of before intervention, after the first intervention, and after the second intervention. The level of significance was set at less than 0.05.
Demographic and menstrual characteristics information of the participants before
intervention can be seen in Table 1. According to the table, the mean and SD
(standard deviation) of age, weight, body mass index, menarche age, duration of
menstruation, and dysmenorrhea self-care did not display a significant difference
between the three groups (p
| Variable (Groups) | Group A | Group B | Group C |
| (Rosa Foetida) | (Placebo) | (Self-care) | |
| Mean |
Mean |
Mean | |
| Age (years) | 21.37 |
21.56 |
21.33 |
| Weight (kg) | 58.68 |
59.31 |
60.20 |
| BMI (kg/m |
22.02 |
22.26 |
22.81 |
| Menarche or age of onset of menstruation (years) | 12.80 |
12.84 |
13.17 |
| Menstruation duration (days) | 6.60 |
6.28 |
6.15 |
| Dysmenorrhea Self-care | 64.12 |
70.64 |
71.28 |
BMI, body mass index; SD, standard deviation.
Table 2 displays the menstrual pain intensity in the participants of the three
groups, before and after the intervention. As it is evident, the pain intensity
of the three groups was significantly different, prior to the intervention; such
that the first group (Rosa Foetida intervention group) had higher pain intensity
(p
| Time (Groups) | Group A | Group B | Group C | p-value |
| (Rosa Foetida) | (Placebo) | (Self-care) | ||
| Mean |
Mean |
Mean | ||
| Before the intervention | 7.200 |
6.51 |
6.044 |
|
| First intervention | 4.24 |
3.94 |
3.00 |
|
| Second intervention | 3.85 |
3.75 |
3.00 |
0.059 |
| p-value |
0.545 |
€, repeated measurement ANOVA; ᵹ, One Way ANOVA Test; Ω, Comparison of
Interaction between time and Groups with repeated measurement ANOVA Tests.
Similar superscripted lowercase letters in each column indicate no significant
difference between different times in each group in the level of
Table 2 exhibited no significant difference between the three groups, although each group showed significant reduction in dysmenorrhea. The Menstrual distress levels were not significantly different in the three groups before the intervention and were significantly decreased after the intervention. As Table 3 exhibits the difference was statistically significant in the three groups; group A experienced a greater decrease in menstrual distress, proving that Rosa Foetida has a greater effect on menstrual distress.
| Time (Groups) | Group A | Group B | Group C | p-value |
| (Rosa Foetida) | (Placebo) | (Self-care) | ||
| Mean |
Mean |
Mean | ||
| Before the intervention | 22.84 |
21.62 |
20.37 |
0.447 |
| First intervention | 19.16 |
18.00 |
18.83 |
0.982 |
| Second intervention | 15.60 |
16.37 |
18.17 |
0.473 |
| p-value |
0.004 | 0.004 | 0.033 |
€, repeated measurement ANOVA; ᵹ, One Way ANOVA; Ω, Comparison of Interaction
between time and Groups with repeated measurement ANOVA Tests. Similar
superscripted lowercase letters in each column indicate no significant difference
between different times in each group in the level of
As it can be seen in Table 4, Group A had a greater blood flow in comparison to
the other two groups, prior to the interventions; however, the difference was not
statistically significant. It is proven that blood flow is reduced in all the
three groups, although the reduction was significant in the case of group A only
(p = 0.041), but the difference was not significant in the three groups
(p
| Time (Groups) | Group A | Group B | Group C | p-value |
| (Rosa Foetida) | (Placebo) | (Self-care) | ||
| Mean |
Mean |
Mean | ||
| Before the intervention | 94.13 |
88.44 |
63.80 |
0.069 |
| First intervention | 69.92 |
84.05 |
71.66 |
0.474 |
| Second intervention | 64.74 |
81.32 |
66.94 |
0.322 |
| p-value |
0.041 | 0.465 | 0.623 | 0.142 |
€, repeated measurement ANOVA; ᵹ, One Way ANOVA; Ω, Comparison of Interaction between time and Groups with repeated measurement ANOVA Tests. Similar superscripted lowercase
letters in each column indicate no significant difference between different times
in each group in the level of
Absenteeism was also reduced in all three groups; however, there were no significant differences between the three groups according to the number of days of absenteeism.
None of the participants exhibited adverse effects due to Rosa Foetida. The intake of painkillers was also reduced in all three groups.
The Rosa Foetida group was also the most content with the treatment. As reported, 26.9% and 45.71% of the participants were content with the treatment in the first and second cycle interventions, respectively. The number of the pain killers which the participants had to take was reduced in all three groups. Although the reduction of need to use pain killer were lower in Rosa Foetida group, the differences were not statistically significant.
This study was conducted to evaluate the effects of Rosa Foetida extract along with menstrual self-care on primary dysmenorrhea, menstrual distress and menstruation blood flow among dysmenorrheic girls.
The results revealed that Rosa Foetida along with self-care could not alleviate primary dysmenorrhea significantly. Similarly, a recent systematic review study indicated that oral intake of Rosa damascene reduced pain severity non-significantly [36]. Another, study also mentioned that administration of Rosa Damascena could not reduce menstrual pain [37]. While, Bani et al. [26] found pain-relieving effects of Rosa Damascena, another study result indicated that the consumption of Rose extract prior to cesarean section reduced surgery pain and the need for painkillers [38].
However, our findings exhibited the significant reduction of menstrual distress. Menstrual distress includes systemic symptoms which could be due to psychological, endocrinal, and cervical factors, as well as increased uterus activity and overproduction of prostaglandins. The Persian yellow rose, is of the rose family and the most important chemicals found in these petals are Vitamins, Fennels, and Flavonoids. Clinical studies on animals as well as humans showed that Fennels and flavonoids offer a multitude of biological effects, such as anti-microbial, anti-tumor, anti-pain, and anti-inflammatory effects [38, 39]. Other interventions have assessed the effectiveness of reducing the menstrual distress. The findings of a recent meta-analysis indicated that the administration of Rosa. Damascene had significant alleviating effects on menstruation-related bloating, fatigue and headache [37]. Another systematic review also reported reducing stress, anxiety and depression among adults [40]. There also were studies that exhibited significant decrease in systemic symptoms following the consumption of Rosa extract [41, 42].
In our study, the self-care group also experienced a reduction in pain intensity, which indicates the effects of exercise and self-care behavior on primary dysmenorrhea. Exercise causes an increase in blood flow which in turn leads to lower amounts of prostaglandins and metabolites in the uterus which reduces pain. Other studies exhibited that moderate to severe exercise intensity may decrease pain by increasing anti-inflammatory cytokines [43]. Furthermore, several studies reported that factors such as self-care behaviors, along with self-management can prove to be effective in primary dysmenorrhea pain reduction [31, 32, 44, 45]. In a clinical trial on three groups (acupressure, self-care and ibuprofen) for pain reduction, researcher found that all three methods reduced the pain. The same can be said in our study as we found a reduction in all three groups. Therefore, the studies mentioned above are confirmative evidences of relieving effects of Rosa extract and are in accordance with our study. Our findings prove the anti-inflammatory effects of Rosa Foetida extract.
In the present study, menstrual blood flow was significantly reduced in the Rosa Foetida group; however, the difference was not statistically significant among the three groups. Davaneghi et al. [46] where the participants were prescribed Rosa damascena extract along with fish oil, bleeding quantity reduction was not reported.
According to the results which showed no significant difference between the three groups in terms of pain reduction, the lack of difference in the number of painkillers consumed, and days of absence from the classes seems reasonable.
To our best knowledge the present study is the first study that assessed Rosa Foetida extract effects combined with self-care on primary dysmenorrhea, menstrual distress, and menstrual bleeding. We suggest further studies to confirm the results.
The consumption of Rosa Foetida extract along with self-care behavior for dysmenorrhea causes a significant reduction in menstrual distress but not in menstrual pain. However, systemic symptoms during menstruation helps women experience relieved menstruation.
VAS, Visual Analogue Scale; MDQ, Menstrual Distress Questionnaire; DSCS, Dysmenorrhea Self Care Scale; TENS, Transcutaneous electrical Nervous System; IRCT, Iranian Registry of Clinical Trial.
The datasets and materials used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
SO and FS contributed to the concept and design of the study; HP, PSK and HN contributed to the design of this study; FS, MF, FNA and ZB collected the data, SK contributed to the data analysis; SK and SO contributed to the interpretation of the data; SO drafted the manuscript and prepared the final version of the manuscript. All authors read and revised the manuscript critically for important intellectual content then approved the final version of the manuscript to be published.
The study design was approved by the ethics Committee of Babol University of Medical Sciences, Babol, Iran (IR.MUBABOL.REC.1397.059). Written informed consent was taken from all the participants. All methods were carried out in accordance with relevant guidelines and regulations.
We would like to thank the Research and Technology Deputy of Babol University of Medical Sciences for the approval and supports; and all the students that participated in the study.
This research was supported by Babol University of Medical Sciences (Grant No. 4984).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
